Question
(4pts) Calculate the moles of Cl in the product (5pts) Enter the empirical formula for your product. BIIIU EI EIE NTI E IT Normal

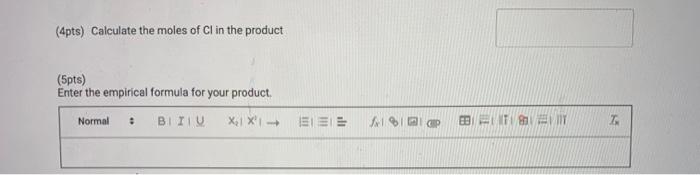
(4pts) Calculate the moles of Cl in the product (5pts) Enter the empirical formula for your product. BIIIU EI EIE NTI E IT Normal
Step by Step Solution
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
a Mass of Mg 5106g 5083g 023g b Mass of product 5174g 5083g 091g c ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting
Authors: Carl S. Warren, James M. Reeve, Jonathan E. Duchac
22nd Edition
324401841, 978-0-324-6250, 0-324-62509-X, 978-0324401844
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



