Answered step by step
Verified Expert Solution
Question
1 Approved Answer
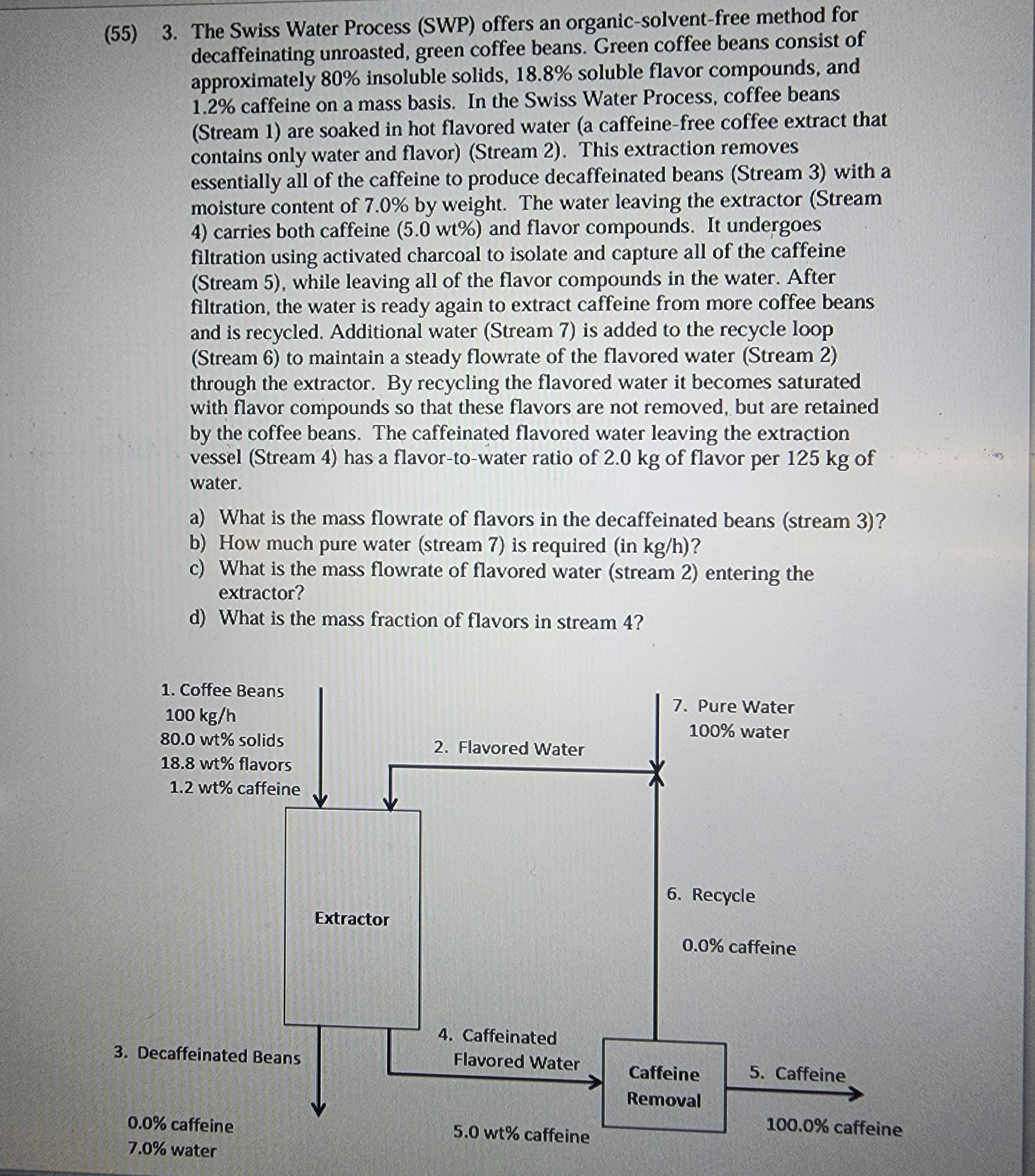
( 5 5 ) 3 . The Swiss Water Process ( SWP ) offers an organic - solvent - free method for decaffeinating unroasted, green
The Swiss Water Process SWP offers an organicsolventfree method for decaffeinating unroasted, green coffee beans. Green coffee beans consist of approximately insoluble solids, soluble flavor compounds, and caffeine on a mass basis. In the Swiss Water Process, coffee beans Stream are soaked in hot flavored water a caffeinefree coffee extract that contains only water and flavorStream This extraction removes essentially all of the caffeine to produce decaffeinated beans Stream with a moisture content of by weight. The water leaving the extractor Stream carries both caffeine and flavor compounds. It undergoes filtration using activated charcoal to isolate and capture all of the caffeine Stream while leaving all of the flavor compounds in the water. After filtration, the water is ready again to extract caffeine from more coffee beans and is recycled. Additional water Stream is added to the recycle loop Stream to maintain a steady flowrate of the flavored water Stream through the extractor. By recycling the flavored water it becomes saturated with flavor compounds so that these flavors are not removed, but are retained by the coffee beans. The caffeinated flavored water leaving the extraction vessel Stream has a flavortowater ratio of of flavor per of water.
a What is the mass flowrate of flavors in the decaffeinated beans stream
b How much pure water stream is required in
c What is the mass flowrate of flavored water stream entering the extractor?
d What is the mass fraction of flavors in stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


