Question
5. A two-dimensional gas is one in which the speeds are distributed only in two directions, such as in the x and y directions.
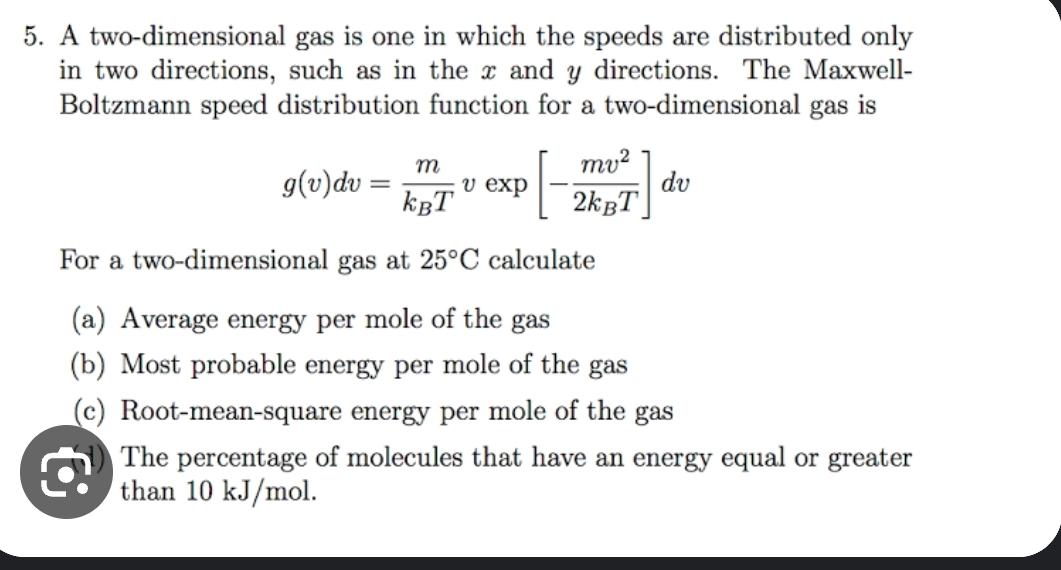
5. A two-dimensional gas is one in which the speeds are distributed only in two directions, such as in the x and y directions. The Maxwell- Boltzmann speed distribution function for a two-dimensional gas is m mv2 g(v)dv= v exp dv KBT 2kBT For a two-dimensional gas at 25C calculate (a) Average energy per mole of the gas (b) Most probable energy per mole of the gas (c) Root-mean-square energy per mole of the gas The percentage of molecules that have an energy equal or greater than 10 kJ/mol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To solve this well use the MaxwellBoltzmann speed distribution function for a twodimensional gas gvdv m m2pi kBT ight v exp mv22kBT ight dv Given that ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Thomas Engel, Philip Reid
3rd edition
805338423, 080533842X, 978-0321812001
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



