Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. The bulk polymerization of styrene at 100C with benzoyl peroxide asthe initiator resulted in a polymer of molecular weight 4.16105. End-use tests showed that
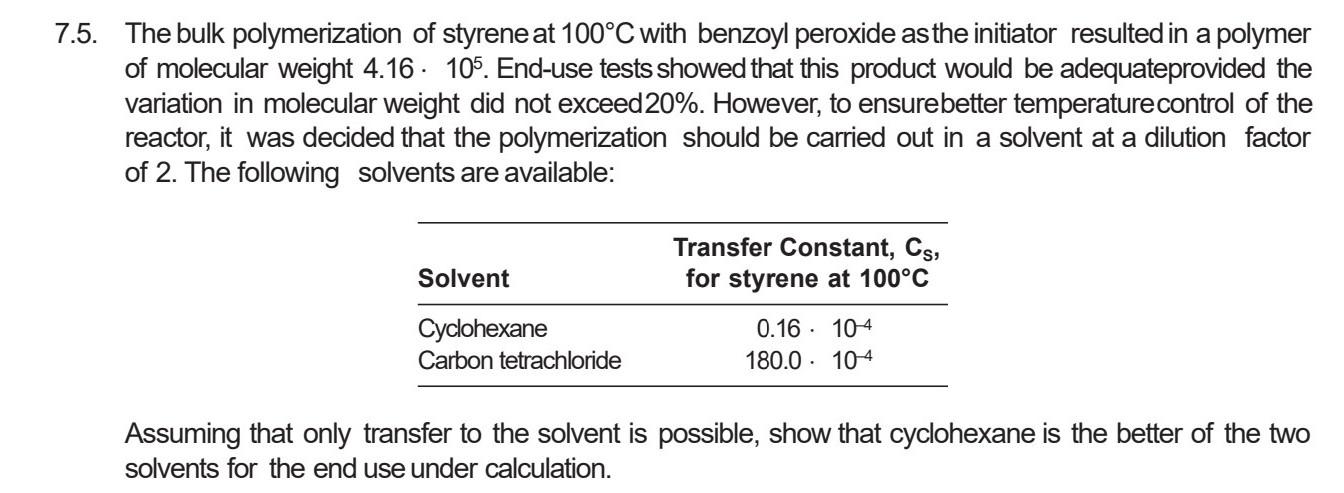
5. The bulk polymerization of styrene at 100C with benzoyl peroxide asthe initiator resulted in a polymer of molecular weight 4.16105. End-use tests showed that this product would be adequateprovided the variation in molecular weight did not exceed 20%. However, to ensurebetter temperaturecontrol of the reactor, it was decided that the polymerization should be carried out in a solvent at a dilution factor of 2 . The following solvents are available: Assuming that only transfer to the solvent is possible, show that cyclohexane is the better of the two solvents for the end use under calculation
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


