Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5-3: Consider a natural gas mixture with mass fractions of methane and ethane of 0.75 and 0.25, respectively. Suppose that a large reservoir contains
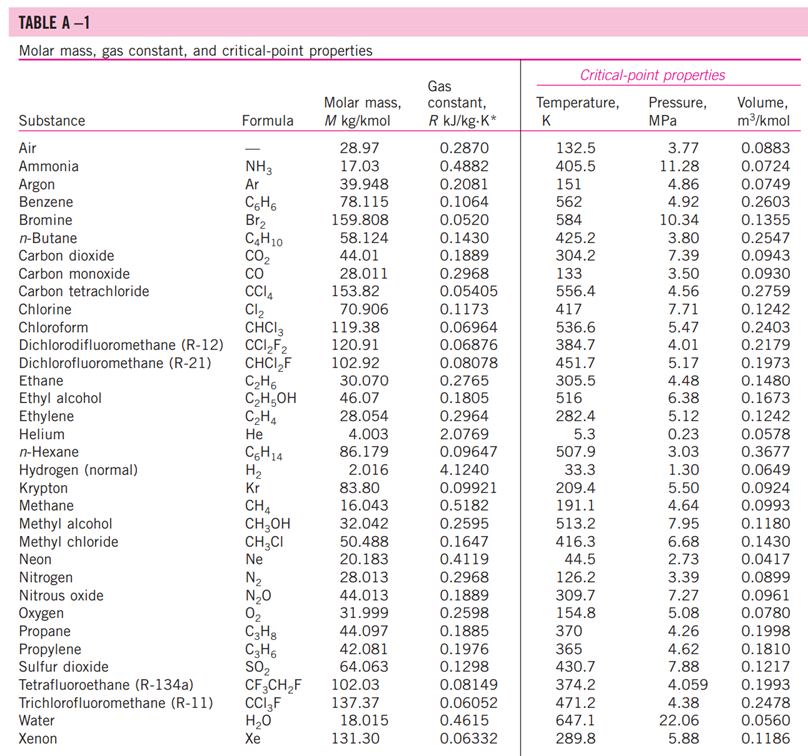
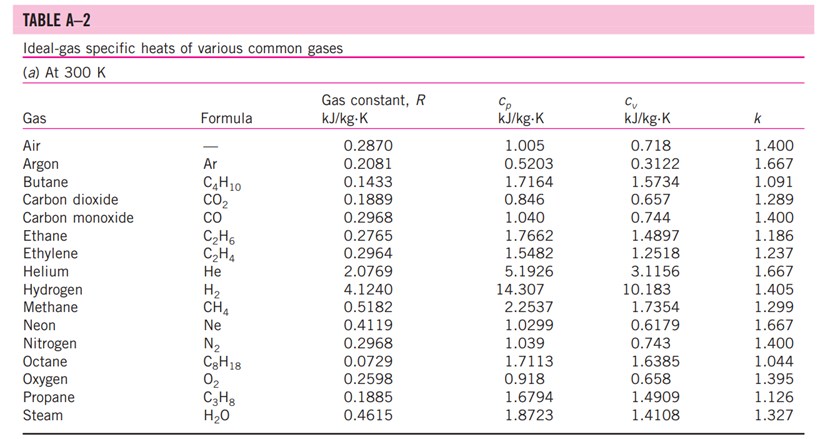
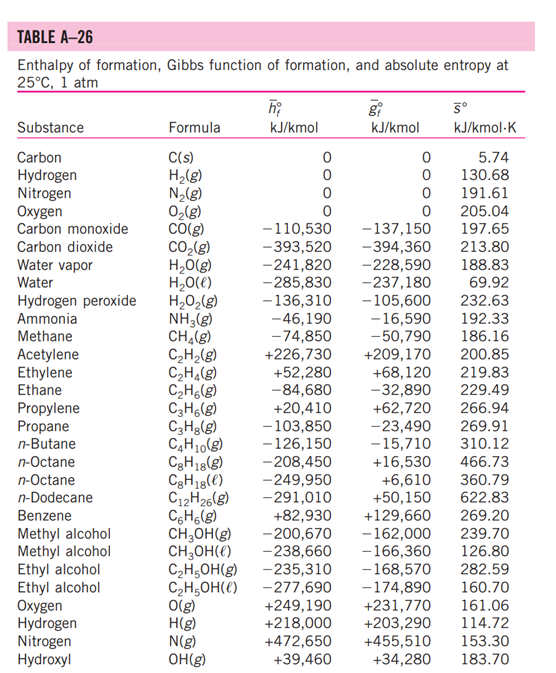
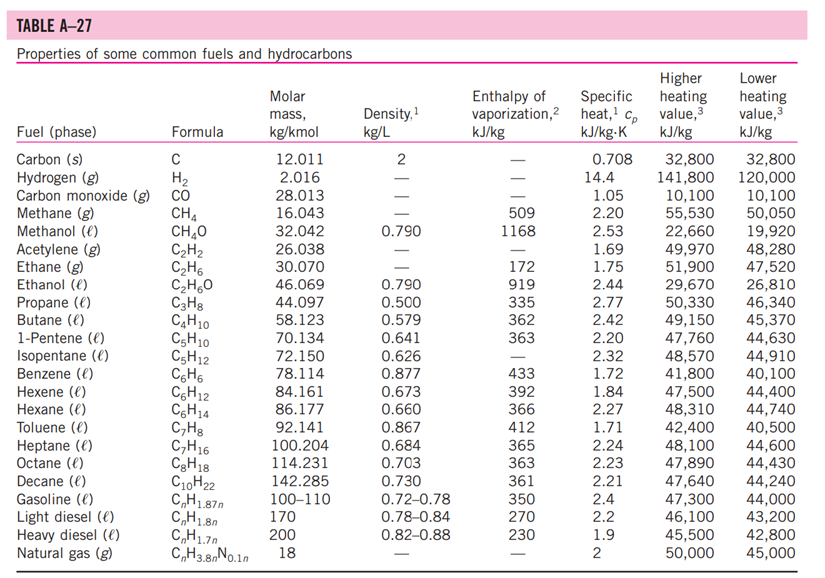
5-3: Consider a natural gas mixture with mass fractions of methane and ethane of 0.75 and 0.25, respectively. Suppose that a large reservoir contains 106 ft of this natural gas at conditions of 2000 psia and 300 deg. F. Find the mass of the gas in the reservoir using the following methods: a. Assuming ideal gas behavior b. Using compressibility factors with Dalton's Law c. Using compressibility factors with Amagat's Law d. Using Kay's Rule TABLE A -1 Molar mass, gas constant, and critical-point properties Substance Air Ammonia Argon Benzene Bromine n-Butane Carbon dioxide Carbon monoxide Carbon tetrachloride Chlorine Chloroform Dichlorodifluoromethane (R-12) Dichlorofluoromethane (R-21) Ethane Ethyl alcohol Ethylene Helium n-Hexane Hydrogen (normal) Krypton Methane Methyl alcohol Methyl chloride Neon Nitrogen Nitrous oxide Oxygen Propane Propylene Sulfur dioxide Tetrafluoroethane (R-134a) Trichlorofluoromethane (R-11) Water Xenon Formula NH3 Ar CH6 Br C4H10 CO CO CCI4 Cl CHCI 3 CClF CHCIF CH6 CH5OH CH4 He C6H14 H Kr CH4 CHOH CH3CI Ne N NO 0 C3H8 C3H6 SO CF3CHF CCI3F HO Xe Molar mass, M kg/kmol 28.97 17.03 39.948 78.115 159.808 58.124 44.01 28.011 153.82 70.906 119.38 120.91 102.92 30.070 46.07 28.054 4.003 86.179 2.016 83.80 16.043 32.042 50.488 20.183 28.013 44.013 31.999 44.097 42.081 64.063 102.03 137.37 18.015 131.30 Gas constant, R kJ/kg-K* 0.2870 0.4882 0.2081 0.1064 0.0520 0.1430 0.1889 0.2968 0.05405 0.1173 0.06964 0.06876 0.08078 0.2765 0.1805 0.2964 2.0769 0.09647 4.1240 0.09921 0.5182 0.2595 0.1647 0.4119 0.2968 0.1889 0.2598 0.1885 0.1976 0.1298 0.08149 0.06052 0.4615 0.06332 Critical-point properties Temperature, Pressure, MPa K 132.5 405.5 151 562 584 425.2 304.2 133 556.4 417 536.6 384.7 451.7 305.5 516 282.4 5.3 507.9 33.3 209.4 191.1 513.2 416.3 44.5 126.2 309.7 154.8 370 365 430.7 374.2 471.2 647.1 289.8 3.77 11.28 4.86 4.92 10.34 3.80 7.39 3.50 4.56 7.71 5.47 4.01 5.17 4.48 6.38 5.12 0.23 3.03 1.30 5.50 4.64 7.95 6.68 2.73 3.39 7.27 5.08 4.26 4.62 7.88 4.059 4.38 22.06 5.88 Volume, m/kmol 0.0883 0.0724 0.0749 0.2603 0.1355 0.2547 0.0943 0.0930 0.2759 0.1242 0.2403 0.2179 0.1973 0.1480 0.1673 0.1242 0.0578 0.3677 0.0649 0.0924 0.0993 0.1180 0.1430 0.0417 0.0899 0.0961 0.0780 0.1998 0.1810 0.1217 0.1993 0.2478 0.0560 0.1186 TABLE A-2 Ideal-gas specific heats of various common gases (a) At 300 K Gas Air Argon Butane Carbon dioxide Carbon monoxide Ethane Ethylene Helium Hydrogen Methane Neon Nitrogen Octane Oxygen Propane Steam Formula | Ar C4H10 CO CO CH6 CH4 He H CH4 Ne N C8H18 0 C3H8 HO Gas constant, R kJ/kg.K 0.2870 0.2081 0.1433 0.1889 0.2968 0.2765 0.2964 2.0769 4.1240 0.5182 0.4119 0.2968 0.0729 0.2598 0.1885 0.4615 Cp kJ/kg.K 1.005 0.5203 1.7164 0.846 1.040 1.7662 1.5482 5.1926 14.307 2.2537 1.0299 1.039 1.7113 0.918 1.6794 1.8723 Cv kJ/kg-K 0.718 0.3122 1.5734 0.657 0.744 1.4897 1.2518 3.1156 10.183 1.7354 0.6179 0.743 1.6385 0.658 1.4909 1.4108 k 1.400 1.667 1.091 1.289 1.400 1.186 1.237 1.667 1.405 1.299 1.667 1.400 1.044 1.395 1.126 1.327 TABLE A-26 Enthalpy of formation, Gibbs function of formation, and absolute entropy at 25C, 1 atm Substance Carbon Hydrogen Nitrogen Oxygen Carbon monoxide Carbon dioxide Water vapor Water Hydrogen peroxide Ammonia Methane Acetylene Ethylene Ethane Propylene Propane n-Butane n-Octane n-Octane n-Dodecane Benzene Methyl alcohol Methyl alcohol Ethyl alcohol Ethyl alcohol Oxygen Hydrogen Nitrogen Hydroxyl Formula C(s) H(g) N(g) 0(g) CO(g) CO(g) HO(g) HO(l) HO(g) NH3(g) CH(g) CH(g) CH(g) CH6(g) hi kJ/kmol -110,530 -393,520 -241,820 -285,830 -136,310 -46,190 -74,850 +226,730 +52,280 -84,680 C3H5(g) +20,410 C3H(g) -103,850 C4H10(g) -126,150 CgH18(g) 0 0 0 0 -208,450 CgH18(e) -249,950 C12H26(g) -291,010 C6H6(g) CHOH(g) CHOH() O(g) H(g) N(g) OH(g) +82,930 -200,670 -238,660 CH5OH(g) -235,310 CH5OH() -277,690 gi kJ/kmol 0 0 0 5 kJ/kmol-K 5.74 130.68 191.61 205.04 197.65 213.80 188.83 69.92 232.63 -16,590 192.33 -50,790 186.16 +209,170 200.85 +68,120 219.83 -32,890 229.49 +62,720 266.94 -23,490 269.91 -15,710 310.12 +16,530 466.73 +6,610 -137,150 -394,360 -228,590 -237,180 -105,600 360.79 +50, 150 622.83 +129,660 269.20 -162,000 239.70 -166,360 126.80 -168,570 282.59 -174,890 160.70 +249,190 +231,770 161.06 +218,000 +203,290 114.72 +472,650 +455,510 153.30 +39,460 +34,280 183.70 TABLE A-27 Properties of some common fuels and hydrocarbons Fuel (phase) Carbon (s) Hydrogen (g) Carbon monoxide (g) Methane (g) Methanol () Acetylene (g) Ethane (g) Ethanol (0) Propane () Butane () 1-Pentene (1) Isopentane () Benzene (l) Hexene (l) Hexane () Toluene () Heptane () Octane () Decane () Gasoline () Light diesel (1) Heavy diesel (e) Natural gas (g) Formula C H CO CH4 CHO CH CH6 CH6O C3H8 C4H10 C5H10 C5H12 C6H6 C6H12 C6H14 CHg CH16 C8H18 C10H22 CH1.87m C,Hi8n CH1.70 CH3.8 No.10 Molar mass, kg/kmol 12.011 2.016 28.013 16.043 32.042 26.038 30.070 46.069 44.097 58.123 70.134 72.150 78.114 84.161 86.177 92.141 100.204 114.231 142.285 100-110 170 200 18 1 Density, kg/L 2 0.790 0.790 0.500 0.579 0.641 0.626 0.877 0.673 0.660 0.867 0.684 0.703 0.730 0.72-0.78 0.78-0.84 0.82-0.88 Higher Enthalpy of Specific heating vaporization,2 heat, cp value, kJ/kg kJ/kg-K kJ/kg 509 1168 172 919 335 362 363 433 392 366 412 365 363 361 350 270 230 0.708 14.4 1.05 2.20 2.53 1.69 1.75 2.44 2.77 2.42 2.20 2.32 1.72 1.84 2.27 1.71 2.24 2.23 2.21 2.4 2.2 1.9 2 32,800 141,800 10,100 55,530 22,660 49,970 51,900 29,670 50,330 49,150 47,760 48,570 41,800 47,500 48,310 42,400 48,100 47,890 47,640 47,300 46,100 45,500 50,000 Lower heating value, kJ/kg 32,800 120,000 10,100 50,050 19,920 48,280 47,520 26,810 46,340 45,370 44,630 44,910 40,100 44,400 44,740 40,500 44,600 44,430 44,240 44,000 43,200 42,800 45,000
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the mass of the gas in the reservoir using different methods well follow the given steps a Assuming ideal gas behavior To calculate the mass o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


