Answered step by step
Verified Expert Solution
Question
1 Approved Answer
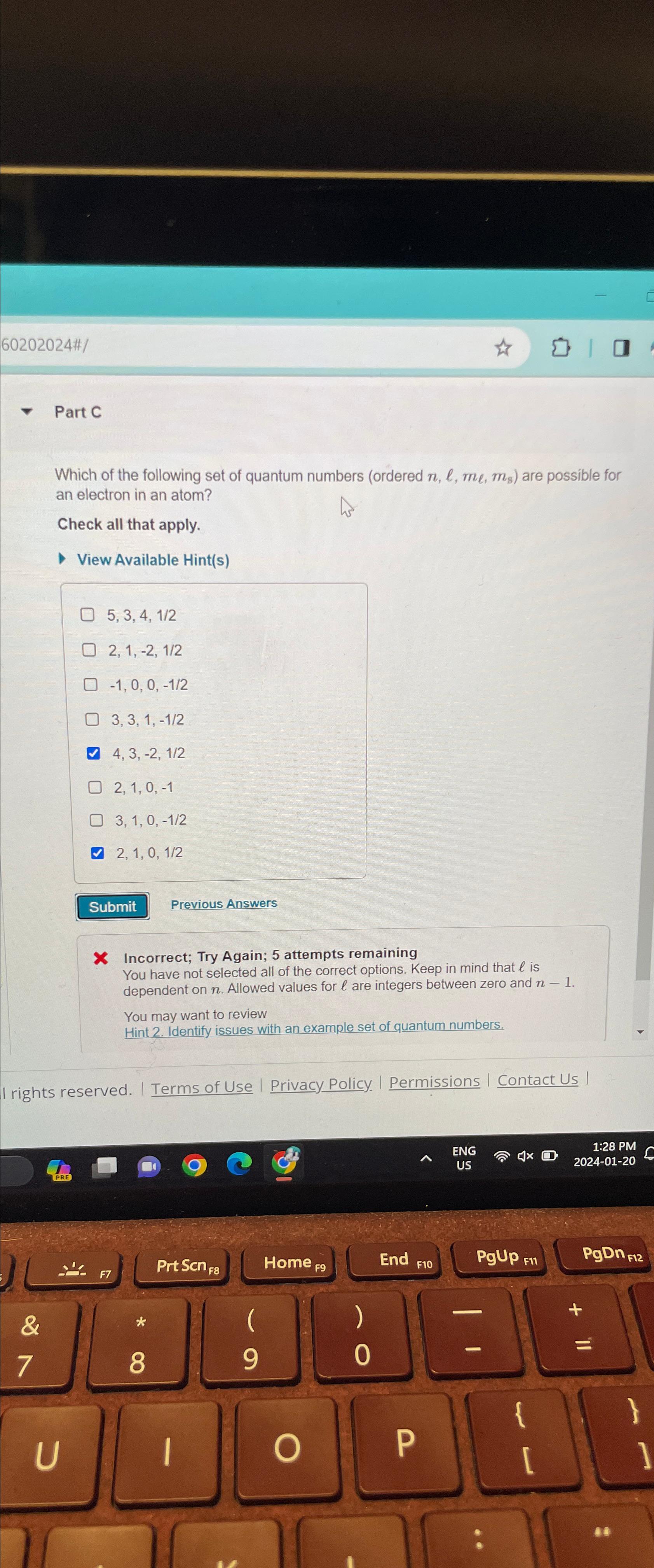
6 0 2 0 2 0 2 4 # | Part C Which of the following set of quantum numbers ( ordered n , l
#
Part C
Which of the following set of quantum numbers ordered are possible for an electron in an atom?
Check all that apply.
View Available Hints
Previous Answers
X Incorrect; Try Again; attempts remaining
You have not selected all of the correct options. Keep in mind that is dependent on Allowed values for are integers between zero and
You may want to review
Hint Identify issues with an example set of quantum numbers.
rights reserved. Terms of Use Privacy Policy Permissions Contact Us
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


