Question
6. (a) State the difference(s) between surface oxidation and corrosion of metals. (b) Describe the Pilling-Bedworth ratio. (c) Calculate the Pilling-Bedworth ratio for tin
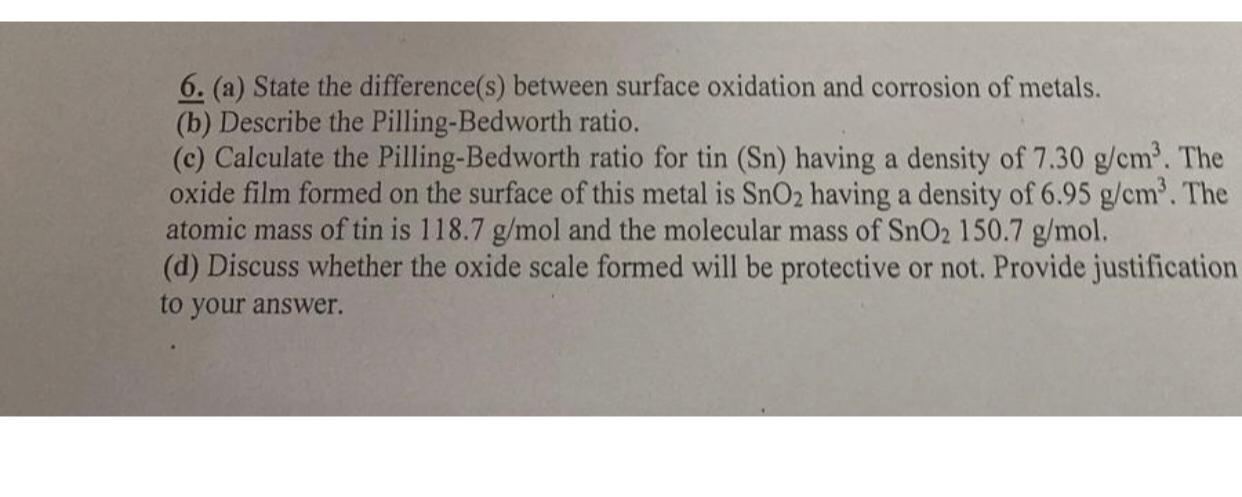
6. (a) State the difference(s) between surface oxidation and corrosion of metals. (b) Describe the Pilling-Bedworth ratio. (c) Calculate the Pilling-Bedworth ratio for tin (Sn) having a density of 7.30 g/cm. The oxide film formed on the surface of this metal is SnO2 having a density of 6.95 g/cm. The atomic mass of tin is 118.7 g/mol and the molecular mass of SnO2 150.7 g/mol. (d) Discuss whether the oxide scale formed will be protective or not. Provide justification to your answer.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



