Answered step by step
Verified Expert Solution
Question
1 Approved Answer
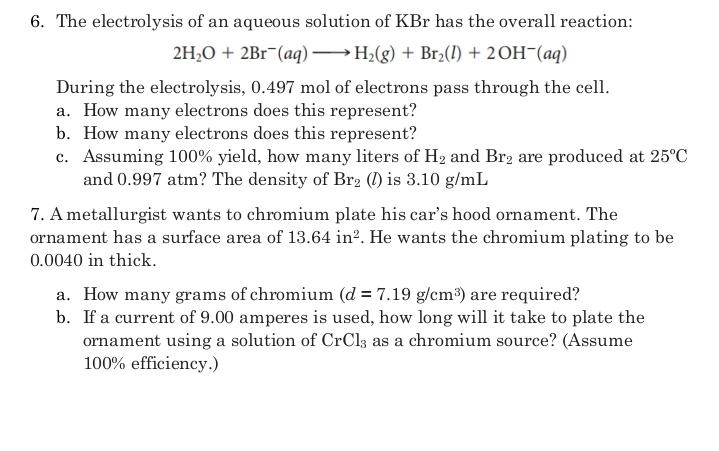
6. The electrolysis of an aqueous solution of KBr has the overall reaction: 2H,0 + 2Br (aq) H;(g) + Br2(1) + 20H-(aq) During the
6. The electrolysis of an aqueous solution of KBr has the overall reaction: 2H,0 + 2Br (aq) H;(g) + Br2(1) + 20H-(aq) During the electrolysis, 0.497 mol of electrons pass through the cell. a. How many electrons does this represent? b. How many electrons does this represent? c. Assuming 100% yield, how many liters of H2 and Br2 are produced at 25C and 0.997 atm? The density of Br2 () is 3.10 g/mL 7. A metallurgist wants to chromium plate his car's hood ornament. The ornament has a surface area of 13.64 in?. He wants the chromium plating to be 0.0040 in thick. a. How many grams of chromium (d = 7.19 g/cm) are required? b. If a current of 9.00 amperes is used, how long will it take to plate the ornament using a solution of CrCl3 as a chromium source? (Assume 100% efficiency.)
Step by Step Solution
★★★★★
3.35 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
6 7 X Step 1 of 4 a Write the overall cell reaction 2H0 2B1 aq Hg Br 1 2...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started