Question
7. (6 points) A monatomic ideal gas is taken through the following three process cycle in a sealed container. The heat entering and leaving
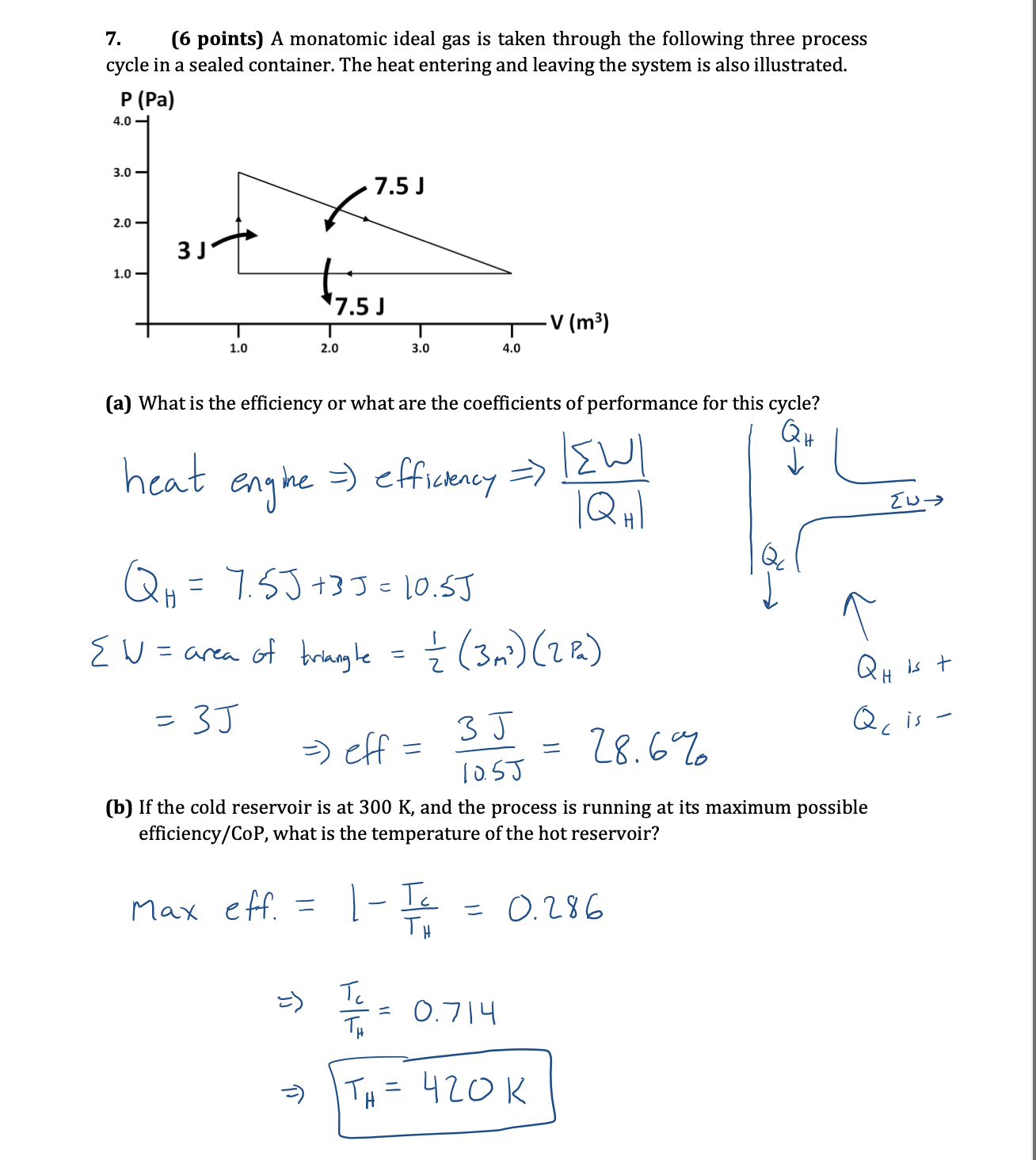
7. (6 points) A monatomic ideal gas is taken through the following three process cycle in a sealed container. The heat entering and leaving the system is also illustrated. P (Pa) 4.0 3.0- 2.0- 1.0- 3 J 1.0 2.0 7.5 J 7.5 J -V (m) 3.0 4.0 (a) What is the efficiency or what are the coefficients of performance for this cycle? heat engine =) efficiency => |Q H Qc Q = 7.5J +33 = 10.5J {W = area of triangle = (3 m) (2 P) = 3J => eff = = 10.5J 28.6% QH is t Quis (b) If the cold reservoir is at 300 K, and the process is running at its maximum possible efficiency/CoP, what is the temperature of the hot reservoir? Max eff = T = 0.286 TT TH TH = 0.714 T = 420K TH
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Optics
Authors: Eugene Hecht
5th edition
133977226, 133979121, 978-0133977226
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



