Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7. Consider the following data: AH (kJ/mole) AG (kJ/mole) CH(g) HO(1) -84.7 -285.9 -32.9 -237.2 CO(g) -393.5 -394.4 a) Calculate the entropy change for
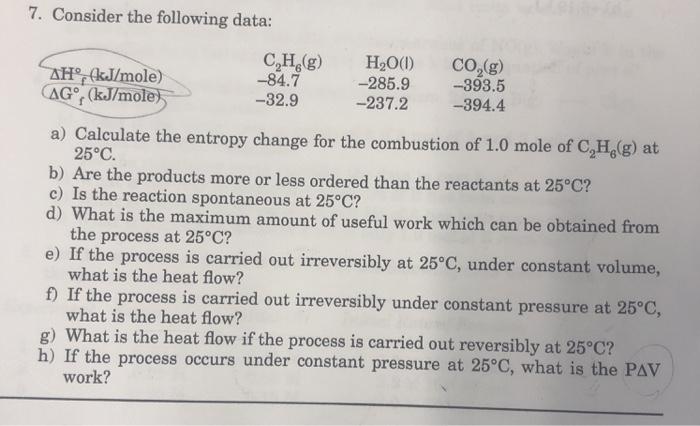
7. Consider the following data: AH (kJ/mole) AG (kJ/mole) CH(g) HO(1) -84.7 -285.9 -32.9 -237.2 CO(g) -393.5 -394.4 a) Calculate the entropy change for the combustion of 1.0 mole of CH(g) at 25C. b) Are the products more or less ordered than the reactants at 25C? c) Is the reaction spontaneous at 25C? d) What is the maximum amount of useful work which can be obtained from the process at 25C? e) If the process is carried out irreversibly at 25C, under constant volume, what is the heat flow? f) If the process is carried out irreversibly under constant pressure at 25C, what is the heat flow? g) What is the heat flow if the process is carried out reversibly at 25C? h) If the process occurs under constant pressure at 25C, what is the PAV work?
Step by Step Solution
★★★★★
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


