Question
7. The formation constant (K) for Cu(NH 3) 4^2+ is 5 * 10 ^ 13 A) Write the balanced reaction. Cu ^2 + (a
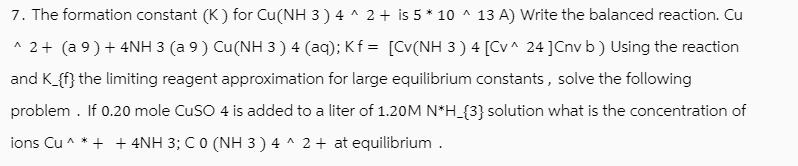
7. The formation constant (K) for Cu(NH 3) 4^2+ is 5 * 10 ^ 13 A) Write the balanced reaction. Cu ^2 + (a 9 ) + 4NH 3 (a 9 ) Cu(NH 3 ) 4 (aq); Kf = [Cv(NH 3) 4 [Cv^ 24 ] Cnv b) Using the reaction and K_{f} the limiting reagent approximation for large equilibrium constants, solve the following problem. If 0.20 mole CuSO 4 is added to a liter of 1.20M N*H_{3} solution what is the concentration of ions Cu^ * + + 4NH 3; C 0 (NH 3 ) 4^2+ at equilibrium.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



