Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7. The solubility product of calcium fluoride (CaF2) is 3.451011. If 1.5mL of a 0.10M solution of NaF is added to 130mL of a 1.0105M
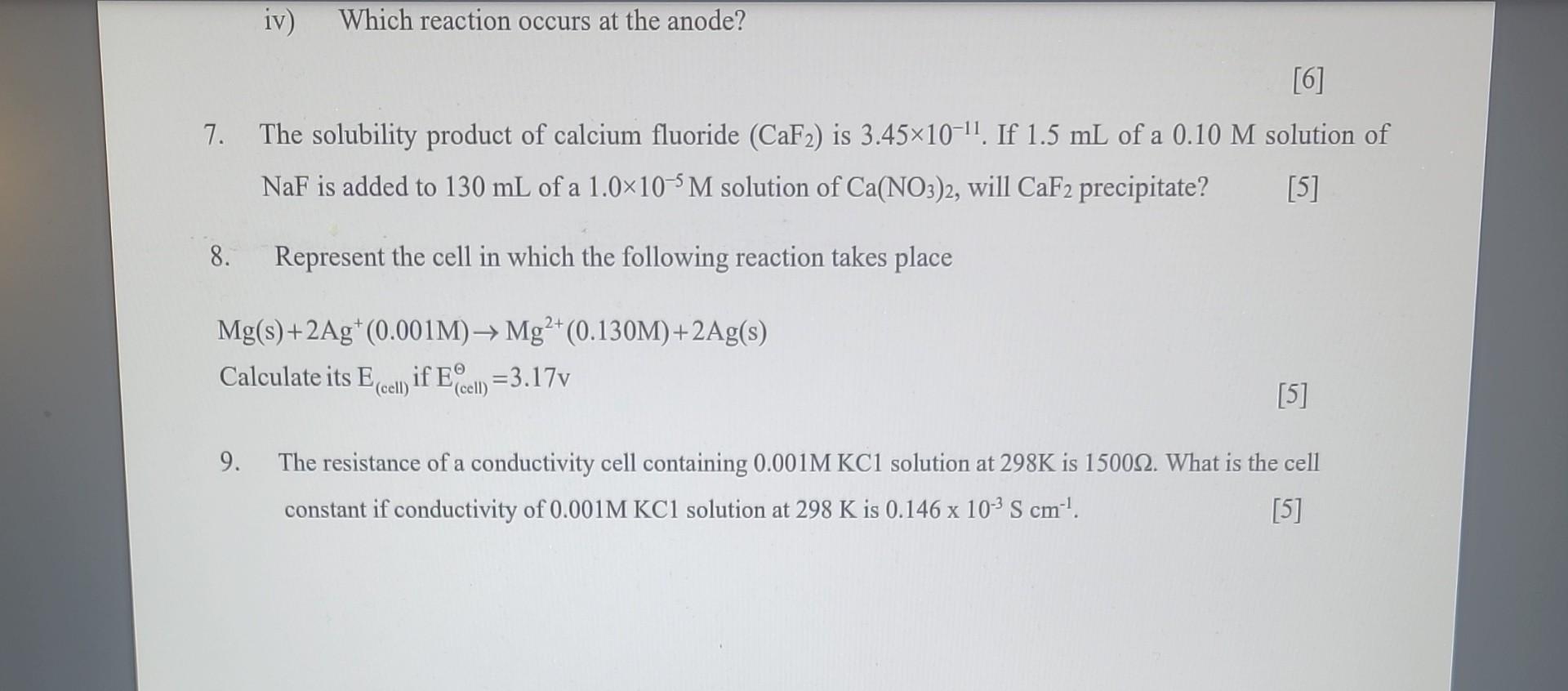
7. The solubility product of calcium fluoride (CaF2) is 3.451011. If 1.5mL of a 0.10M solution of NaF is added to 130mL of a 1.0105M solution of Ca(NO3)2, will CaF2 precipitate? [5] 8. Represent the cell in which the following reaction takes place Mg(s)+2Ag+(0.001M)Mg2+(0.130M)+2Ag(s) Calculate its E(cell) if E(cell)=3.17v 9. The resistance of a conductivity cell containing 0.001MKC1 solution at 298K is 1500. What is the cell constant if conductivity of 0.001MKC1 solution at 298K is 0.146103Scm1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


