Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(7.47 of Koretsky 2nd edition) A binary mixture of species 1 and 2 can be described by the following equation of state: P =
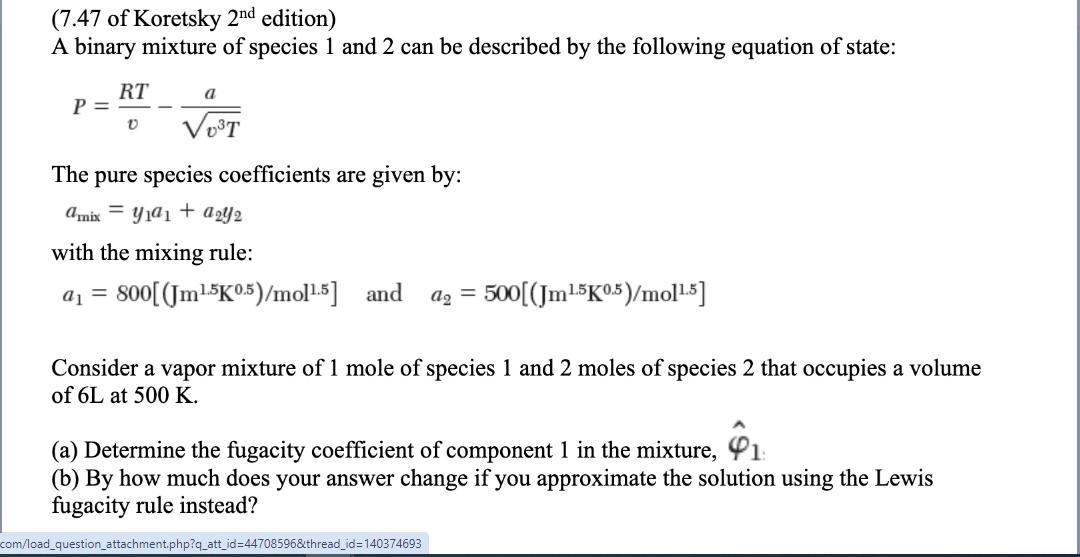
(7.47 of Koretsky 2nd edition) A binary mixture of species 1 and 2 can be described by the following equation of state: P = a 0 VoT The pure species coefficients are given by: amix y1a1a2Y2 with the mixing rule: a =800[(Jm1.5K 0.5)/mol1.5] and a2 = 500[(Jm15K0.5)/mol-5] Consider a vapor mixture of 1 mole of species 1 and 2 moles of species 2 that occupies a volume of 6L at 500 K. (a) Determine the fugacity coefficient of component 1 in the mixture, (b) By how much does your answer change if you approximate the solution using the Lewis fugacity rule instead? com/load_question_attachment.php?q_att_id=44708596&thread_id=140374693
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


