Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8. (a) A mixture of propane and butane is burned with pure 02. The combustion products contain 47.4 mole % H20. After all the water
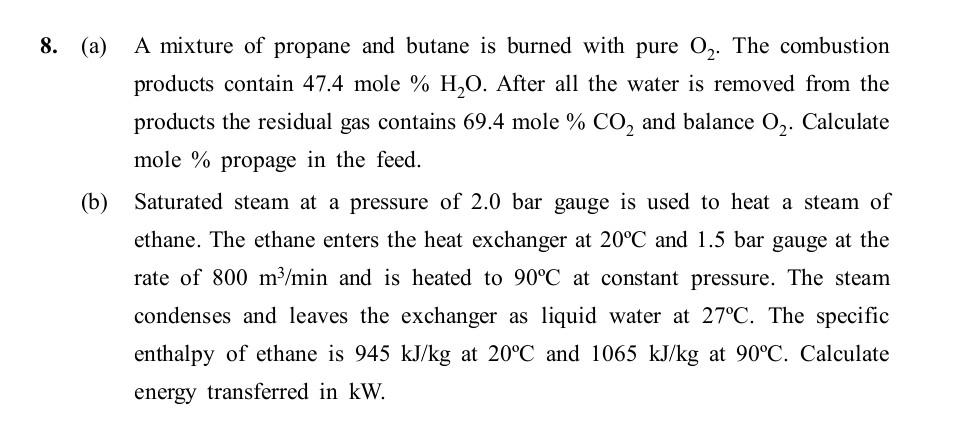
8. (a) A mixture of propane and butane is burned with pure 02. The combustion products contain 47.4 mole % H20. After all the water is removed from the products the residual gas contains 69.4 mole % CO2 and balance 02. Calculate mole % propage in the feed. (b) Saturated steam at a pressure of 2.0 bar gauge is used to heat a steam of ethane. The ethane enters the heat exchanger at 20C and 1.5 bar gauge at the rate of 800 m/min and is heated to 90C at constant pressure. The steam condenses and leaves the exchanger as liquid water at 27C. The specific enthalpy of ethane is 945 kJ/kg at 20C and 1065 kJ/kg at 90C. Calculate energy transferred in kW
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


