Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8. Stepwise polymerization reaction commonly proceeds by a condensation reaction and chain reaction proceeds by the chain carriers. (a) Which reaction rate is usually faster?
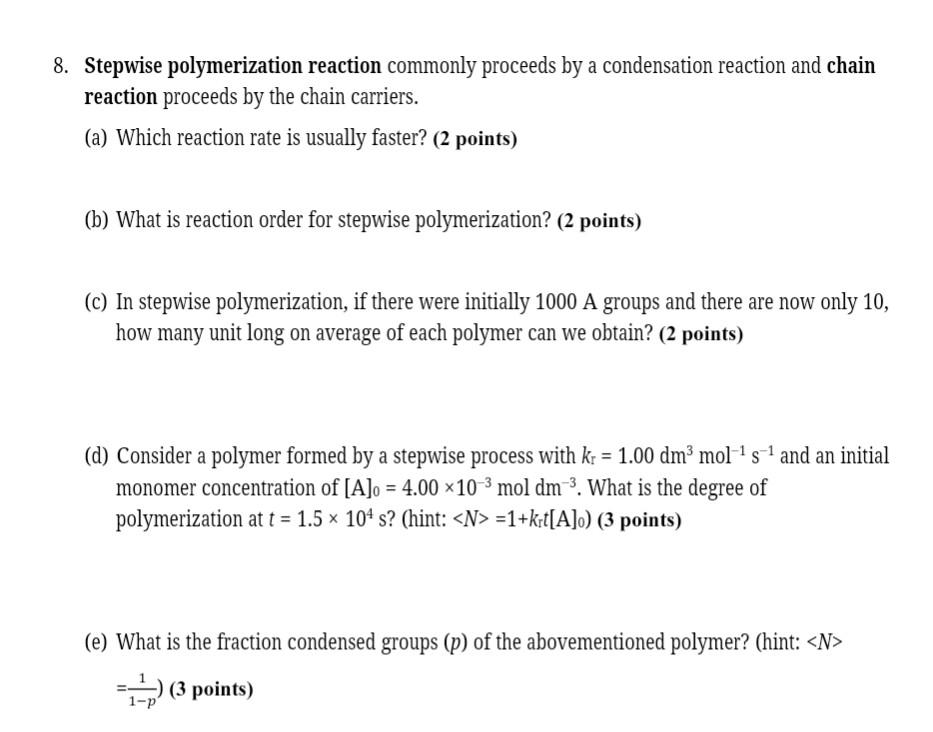
8. Stepwise polymerization reaction commonly proceeds by a condensation reaction and chain reaction proceeds by the chain carriers. (a) Which reaction rate is usually faster? (2 points) (b) What is reaction order for stepwise polymerization? (2 points) (C) In stepwise polymerization, if there were initially 1000 A groups and there are now only 10, how many unit long on average of each polymer can we obtain? (2 points) (d) Consider a polymer formed by a stepwise process with k = 1.00 dm3 mol's-1 and an initial monomer concentration of [A]o = 4.00 x 10-3 mol dm? What is the degree of polymerization at t = 1.5 x 104 s? (hint:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


