Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-116 Steam enters a two-stage adiabatic turbine at 8 MPa and 500C. It expands in the first stage to a state of 2 MPa
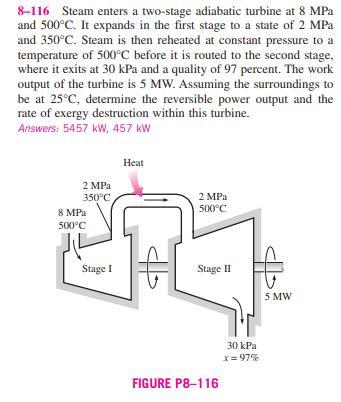

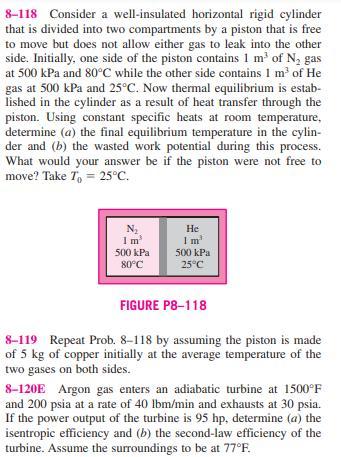
8-116 Steam enters a two-stage adiabatic turbine at 8 MPa and 500C. It expands in the first stage to a state of 2 MPa and 350C. Steam is then reheated at constant pressure to a temperature of 500C before it is routed to the second stage, where it exits at 30 kPa and a quality of 97 percent. The work output of the turbine is 5 MW. Assuming the surroundings to be at 25C, determine the reversible power output and the rate of exergy destruction within this turbine. Answers: 5457 kW, 457 KW 8 MPa 2 MPa 350C 500C Heat 2 MPa 500C Stage I Stage II FIGURE P8-116 30 kPa x=97% 5 MW 8-117 One ton of liquid water at 80C is brought into a well-insulated and well-sealed 4-m X 5-m X 6-m room ini- tially at 22C and 100 kPa. Assuming constant specific heats for both the air and water at room temperature, determine (a) the final equilibrium temperature in the room, (b) the exergy destruction, (c) the maximum amount of work that can be produced during this process, in kJ. Take T = 10C. 8-118 Consider a well-insulated horizontal rigid cylinder that is divided into two compartments by a piston that is free to move but does not allow either gas to leak into the other side. Initially, one side of the piston contains 1 m of N, gas at 500 kPa and 80C while the other side contains 1 m of He gas at 500 kPa and 25C. Now thermal equilibrium is estab- lished in the cylinder as a result of heat transfer through the piston. Using constant specific heats at room temperature, determine (a) the final equilibrium temperature in the cylin- der and (b) the wasted work potential during this process. What would your answer be if the piston were not free to move? Take To = 25C. N He I m I m 500 kPa 500 kPa 80C 25C FIGURE P8-118 8-119 Repeat Prob. 8-118 by assuming the piston is made of 5 kg of copper initially at the average temperature of the two gases on both sides. 8-120E Argon gas enters an adiabatic turbine at 1500F and 200 psia at a rate of 40 lbm/min and exhausts at 30 psia. If the power output of the turbine is 95 hp, determine (a) the isentropic efficiency and (b) the second-law efficiency of the turbine. Assume the surroundings to be at 77F. 8-116 Steam enters a two-stage adiabatic turbine at 8 MPa and 500C. It expands in the first stage to a state of 2 MPa and 350C. Steam is then reheated at constant pressure to a temperature of 500C before it is routed to the second stage, where it exits at 30 kPa and a quality of 97 percent. The work output of the turbine is 5 MW. Assuming the surroundings to be at 25C, determine the reversible power output and the rate of exergy destruction within this turbine. Answers: 5457 kW, 457 KW 8 MPa 2 MPa 350C 500C Heat 2 MPa 500C Stage I Stage II FIGURE P8-116 30 kPa x=97% 5 MW 8-117 One ton of liquid water at 80C is brought into a well-insulated and well-sealed 4-m X 5-m X 6-m room ini- tially at 22C and 100 kPa. Assuming constant specific heats for both the air and water at room temperature, determine (a) the final equilibrium temperature in the room, (b) the exergy destruction, (c) the maximum amount of work that can be produced during this process, in kJ. Take T = 10C. 8-118 Consider a well-insulated horizontal rigid cylinder that is divided into two compartments by a piston that is free to move but does not allow either gas to leak into the other side. Initially, one side of the piston contains 1 m of N, gas at 500 kPa and 80C while the other side contains 1 m of He gas at 500 kPa and 25C. Now thermal equilibrium is estab- lished in the cylinder as a result of heat transfer through the piston. Using constant specific heats at room temperature, determine (a) the final equilibrium temperature in the cylin- der and (b) the wasted work potential during this process. What would your answer be if the piston were not free to move? Take To = 25C. N He I m I m 500 kPa 500 kPa 80C 25C FIGURE P8-118 8-119 Repeat Prob. 8-118 by assuming the piston is made of 5 kg of copper initially at the average temperature of the two gases on both sides. 8-120E Argon gas enters an adiabatic turbine at 1500F and 200 psia at a rate of 40 lbm/min and exhausts at 30 psia. If the power output of the turbine is 95 hp, determine (a) the isentropic efficiency and (b) the second-law efficiency of the turbine. Assume the surroundings to be at 77F.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


