Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-131 Can closed-system exergy be negative? How about flow exergy? Explain using an incompressible substance as an example. 8-132 Obtain a relation for the
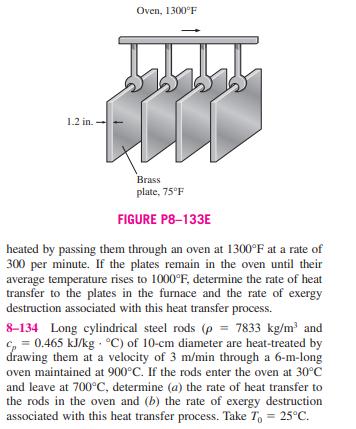

8-131 Can closed-system exergy be negative? How about flow exergy? Explain using an incompressible substance as an example. 8-132 Obtain a relation for the second-law efficiency of a heat engine that receives heat Q from a source at tempera- ture Ty, and rejects heat Q, to a sink at T, which is higher than To (the temperature of the surroundings), while produc- ing work in the amount of W. 8-133E In a production facility, 1.2-in-thick, 2-ft x 2-ft square brass plates (p 532.5 lbm/ft and c = 0.091 Btu/lbm F) that are initially at a uniform temperature of 75F are 1.2 in. Oven, 1300F Brass plate, 75F FIGURE P8-133E heated by passing them through an oven at 1300F at a rate of 300 per minute. If the plates remain in the oven until their average temperature rises to 1000F, determine the rate of heat transfer to the plates in the furnace and the rate of exergy destruction associated with this heat transfer process. 8-134 Long cylindrical steel rods (p = 7833 kg/m and c = 0.465 kJ/kg C) of 10-cm diameter are heat-treated by drawing them at a velocity of 3 m/min through a 6-m-long oven maintained at 900C. If the rods enter the oven at 30C and leave at 700C, determine (a) the rate of heat transfer to the rods in the oven and (b) the rate of exergy destruction associated with this heat transfer process. Take T = 25C. 8-135 Steam is to be condensed in the condenser of a steam power plant at a temperature of 60C with cooling water from a nearby lake that enters the tubes of the condenser at 15C at a rate of 140 kg/s and leaves at 25C. Assuming the condenser to be perfectly insulated, determine (a) the rate of condensa- tion of the steam and (b) the rate of exergy destruction in the condenser. Answers: (a) 2.48 kg, (b) 694 kW 8-131 Can closed-system exergy be negative? How about flow exergy? Explain using an incompressible substance as an example. 8-132 Obtain a relation for the second-law efficiency of a heat engine that receives heat Q from a source at tempera- ture Ty, and rejects heat Q, to a sink at T, which is higher than To (the temperature of the surroundings), while produc- ing work in the amount of W. 8-133E In a production facility, 1.2-in-thick, 2-ft x 2-ft square brass plates (p 532.5 lbm/ft and c = 0.091 Btu/lbm F) that are initially at a uniform temperature of 75F are 1.2 in. Oven, 1300F Brass plate, 75F FIGURE P8-133E heated by passing them through an oven at 1300F at a rate of 300 per minute. If the plates remain in the oven until their average temperature rises to 1000F, determine the rate of heat transfer to the plates in the furnace and the rate of exergy destruction associated with this heat transfer process. 8-134 Long cylindrical steel rods (p = 7833 kg/m and c = 0.465 kJ/kg C) of 10-cm diameter are heat-treated by drawing them at a velocity of 3 m/min through a 6-m-long oven maintained at 900C. If the rods enter the oven at 30C and leave at 700C, determine (a) the rate of heat transfer to the rods in the oven and (b) the rate of exergy destruction associated with this heat transfer process. Take T = 25C. 8-135 Steam is to be condensed in the condenser of a steam power plant at a temperature of 60C with cooling water from a nearby lake that enters the tubes of the condenser at 15C at a rate of 140 kg/s and leaves at 25C. Assuming the condenser to be perfectly insulated, determine (a) the rate of condensa- tion of the steam and (b) the rate of exergy destruction in the condenser. Answers: (a) 2.48 kg, (b) 694 kW
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


