Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-21 How much of the 100 kJ of thermal energy at 800 K can be converted to useful work? Assume the environment to be
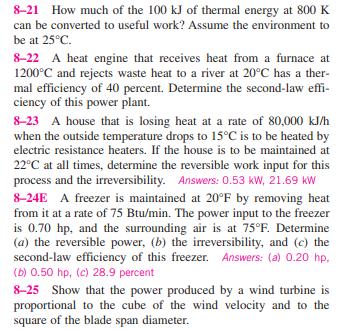
8-21 How much of the 100 kJ of thermal energy at 800 K can be converted to useful work? Assume the environment to be at 25C. 8-22 A heat engine that receives heat from a furnace at 1200C and rejects waste heat to a river at 20C has a ther- mal efficiency of 40 percent. Determine the second-law effi- ciency of this power plant. 8-23 A house that is losing heat at a rate of 80,000 kJ/h when the outside temperature drops to 15C is to be heated by electric resistance heaters. If the house is to be maintained at 22C at all times, determine the reversible work input for this process and the irreversibility. Answers: 0.53 kW, 21.69 kW 8-24E A freezer is maintained at 20F by removing heat from it at a rate of 75 Btu/min. The power input to the freezer is 0.70 hp, and the surrounding air is at 75F. Determine (a) the reversible power, (b) the irreversibility, and (c) the second-law efficiency of this freezer. Answers: (a) 0.20 hp, (b) 0.50 hp, (c) 28.9 percent 8-25 Show that the power produced by a wind turbine is proportional to the cube of the wind velocity and to the square of the blade span diameter.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


