Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-46 A 50-kg iron block and a 20-kg copper block, both initially at 80C, are dropped into a large lake at 15C. Ther- mal
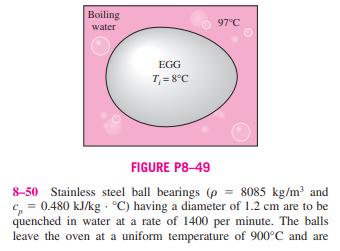

8-46 A 50-kg iron block and a 20-kg copper block, both initially at 80C, are dropped into a large lake at 15C. Ther- mal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Assuming the surroundings to be at 20C, determine the amount of work that could have been produced if the entire process were exe- cuted in a reversible manner. 8-47E A 12-ft rigid tank contains refrigerant-134a at 40 psia and 55 percent quality. Heat is transferred now to the refrigerant from a source at 120F until the pressure rises to 60 psia. Assuming the surroundings to be at 75F, determine (a) the amount of heat transfer between the source and the refrigerant and (b) the exergy destroyed during this process. 8-48 Chickens with an average mass of 2.2 kg and average specific heat of 3.54 kJ/kg C are to be cooled by chilled water that enters a continuous-flow-type immersion chiller at 0.5C and leaves at 2.5C. Chickens are dropped into the chiller at a uniform temperature of 15C at a rate of 500 chick- ens per hour and are cooled to an average temperature of 3C before they are taken out. The chiller gains heat from the sur- roundings at a rate of 200 kJ/h. Determine (a) the rate of heat removal from the chicken, in kW, and (b) the rate of exergy destruction during this chilling process. Take To = 25C. 8-49 An ordinary egg can be approximated as a 5.5-cm- diameter sphere. The egg is initially at a uniform temperature of 8C and is dropped into boiling water at 97C. Taking the prop- erties of egg to be p = 1020 kg/m and c, = 3.32 kJ/kg C, determine how much heat is transferred to the egg by the time the average temperature of the egg rises to 70C and the amount of exergy destruction associated with this heat transfer process. Take To = 25C. Boiling water EGG T = 8C 97C FIGURE P8-49 8-50 Stainless steel ball bearings (p = 8085 kg/m and c=0.480 kJ/kg C) having a diameter of 1.2 cm are to be quenched in water at a rate of 1400 per minute. The balls leave the oven at a uniform temperature of 900C and are exposed to air at 30C for a while before they are dropped into the water. If the temperature of the balls drops to 850C prior to quenching, determine (a) the rate of heat transfer from the balls to the air and (b) the rate of exergy destruction due to heat loss from the balls to the air.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


