Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-81E An insulated 150-ft rigid tank contains air at 75 psia and 140F. A valve connected to the tank is opened, and air is
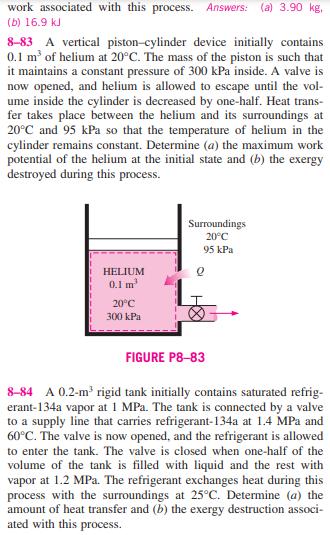

8-81E An insulated 150-ft rigid tank contains air at 75 psia and 140F. A valve connected to the tank is opened, and air is allowed to escape until the pressure inside drops to 30 psia. The air temperature during this process is maintained constant by an electric resistance heater placed in the tank. Determine (a) the electrical work done during this process and (b) the exergy destruction. Assume the surroundings to be at 70F. Answers: (a) 1249 Btu, (b) 1068 Btu 8-82 A 0.1-m rigid tank contains saturated refrigerant- 134a at 800 kPa. Initially, 30 percent of the volume is occu- pied by liquid and the rest by vapor. A valve at the bottom of the tank is opened, and liquid is withdrawn from the tank. Heat is transferred to the refrigerant from a source at 60C so that the pressure inside the tank remains constant. The valve is closed when no liquid is left in the tank and vapor starts to come out. Assuming the surroundings to be at 25C, deter- mine (a) the final mass in the tank and (b) the reversible work associated with this process. Answers: (a) 3.90 kg, (b) 16.9 kJ 8-83 A vertical piston-cylinder device initially contains 0.1 m of helium at 20C. The mass of the piston is such that it maintains a constant pressure of 300 kPa inside. A valve is now opened, and helium is allowed to escape until the vol- ume inside the cylinder is decreased by one-half. Heat trans- fer takes place between the helium and its surroundings at 20C and 95 kPa so that the temperature of helium in the cylinder remains constant. Determine (a) the maximum work potential of the helium at the initial state and (b) the exergy destroyed during this process. HELIUM 0.1 m 20C 300 kPa Surroundings 20C 95 kPa FIGURE P8-83 8-84 A 0.2-m rigid tank initially contains saturated refrig- erant-134a vapor at 1 MPa. The tank is connected by a valve to a supply line that carries refrigerant-134a at 1.4 MPa and 60C. The valve is now opened, and the refrigerant is allowed to enter the tank. The valve is closed when one-half of the volume of the tank is filled with liquid and the rest with vapor at 1.2 MPa. The refrigerant exchanges heat during this process with the surroundings at 25C. Determine (a) the amount of heat transfer and (b) the exergy destruction associ- ated with this process. 8-85 An insulated vertical piston-cylinder device initially contains 15 kg of water, 9 kg of which is in the vapor phase. The mass of the piston is such that it maintains a constant pressure of 200 kPa inside the cylinder. Now steam at 1 MPa and 400C is allowed to enter the cylinder from a supply line until all the liquid in the cylinder is vaporized. Assuming the surroundings to be at 25C and 100 kPa, determine (a) the amount of steam that has entered and (b) the exergy destroyed during this process. Answers: (a) 23.66 kg, (b) 7610 kJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


