Answered step by step
Verified Expert Solution
Question
1 Approved Answer
8-76E A hot-water stream at 160F enters an adiabatic mixing chamber with a mass flow rate of 4 lbm/s, where it is mixed with
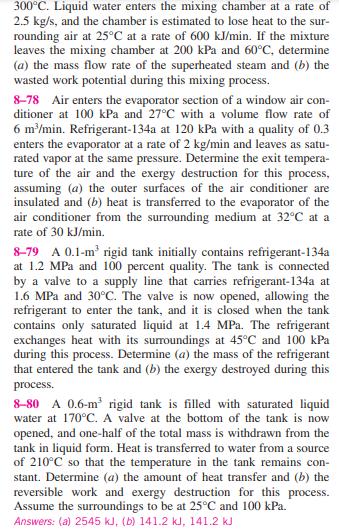
8-76E A hot-water stream at 160F enters an adiabatic mixing chamber with a mass flow rate of 4 lbm/s, where it is mixed with a stream of cold water at 70F. If the mixture leaves the chamber at 110F, determine (a) the mass flow rate of the cold water and (b) the exergy destroyed during this adiabatic mixing process. Assume all the streams are at a pressure of 50 psia and the surroundings are at 75F. Answers: (a) 5.0 lbm/s, (b) 14.6 Btu/s 8-77 Liquid water at 200 kPa and 20C is heated in a chamber by mixing it with superheated steam at 200 kPa and 600 kJ/min 20C 2.5 kg/s Mixing chamber 60C 300C 200 kPa FIGURE P8-77 300C. Liquid water enters the mixing chamber at a rate of 2.5 kg/s, and the chamber is estimated to lose heat to the sur- rounding air at 25C at a rate of 600 kJ/min. If the mixture leaves the mixing chamber at 200 kPa and 60C, determine (a) the mass flow rate of the superheated steam and (b) the wasted work potential during this mixing process. 8-78 Air enters the evaporator section of a window air con- ditioner at 100 kPa and 27C with a volume flow rate of 6 m/min. Refrigerant-134a at 120 kPa with a quality of 0.3 enters the evaporator at a rate of 2 kg/min and leaves as satu- rated vapor at the same pressure. Determine the exit tempera- ture of the air and the exergy destruction for this process, assuming (a) the outer surfaces of the air conditioner are insulated and (b) heat is transferred to the evaporator of the air conditioner from the surrounding medium at 32C at a rate of 30 kJ/min. 8-79 A 0.1-m rigid tank initially contains refrigerant-134a at 1.2 MPa and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant-134a at 1.6 MPa and 30C. The valve is now opened, allowing the refrigerant to enter the tank, and it is closed when the tank contains only saturated liquid at 1.4 MPa. The refrigerant exchanges heat with its surroundings at 45C and 100 kPa during this process. Determine (a) the mass of the refrigerant that entered the tank and (b) the exergy destroyed during this process. 8-80 A 0.6-m rigid tank is filled with saturated liquid water at 170C. A valve at the bottom of the tank is now opened, and one-half of the total mass is withdrawn from the tank in liquid form. Heat is transferred to water from a source of 210C so that the temperature in the tank remains con- stant. Determine (a) the amount of heat transfer and (b) the reversible work and exergy destruction for this process. Assume the surroundings to be at 25C and 100 kPa. Answers: (a) 2545 kJ, (b) 141.2 kJ, 141.2 kJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


