Answered step by step
Verified Expert Solution
Question
1 Approved Answer
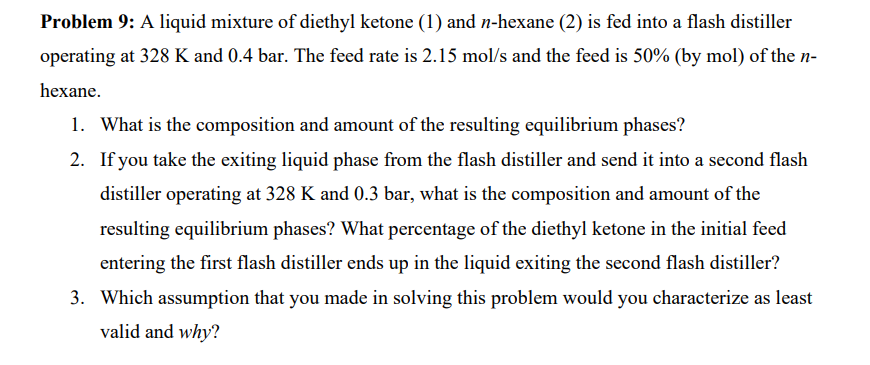
9 : A liquid mixture of diethyl ketone ( 1 ) and n - hexane ( 2 ) is fed into a flash distiller operating
: A liquid mixture of diethyl ketone and hexane is fed into a flash distiller
operating at and The feed rate is and the feed is by mol of the
hexane.
What is the composition and amount of the resulting equilibrium phases?
If you take the exiting liquid phase from the flash distiller and send it into a second flash
distiller operating at and what is the composition and amount of the
resulting equilibrium phases? What percentage of the diethyl ketone in the initial feed
entering the first flash distiller ends up in the liquid exiting the second flash distiller?
Which assumption that you made in solving this problem would you characterize as least
valid and why?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


