Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A = 1 B = 9 X = 0 Y = 5 Z = 3 (Use A,B,X,Y, and Z values from your student number as
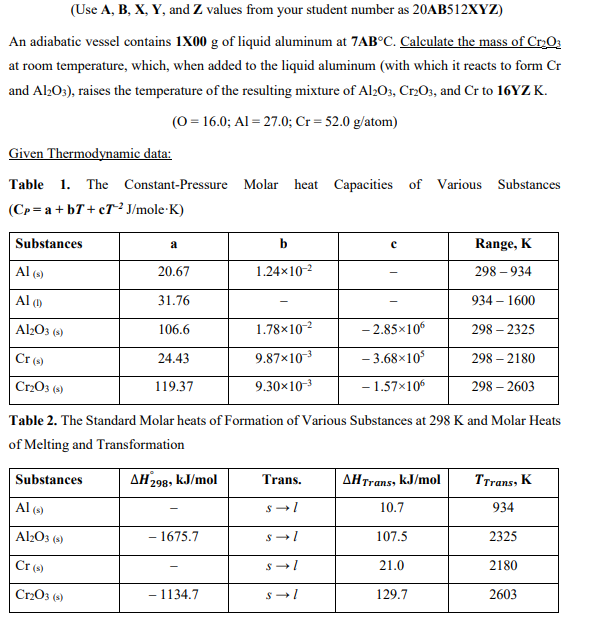
A = 1 B = 9
X = 0 Y = 5 Z = 3
(Use A,B,X,Y, and Z values from your student number as 20AB512XZ ) An adiabatic vessel contains 1X00g of liquid aluminum at 7ABC. Calculate the mass of Cr2O3 at room temperature, which, when added to the liquid aluminum (with which it reacts to form Cr and Al2O3 ), raises the temperature of the resulting mixture of Al2O3,Cr2O3, and Cr to 16YZ K. (O=16.0;Al=27.0;Cr=52.0g/atom) Given Thermodynamic data: Table 1. The Constant-Pressure Molar heat Capacities of Various Substances (CP=a+bT+cT2J/moleK) Table 2. The Standard Molar heats of Formation of Various Substances at 298K and Molar Heats of Melting and TransformationStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


