Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(a) 2. Set up a stoichiometric table for each of the following reactions and express the concentration of each species in the reaction as a
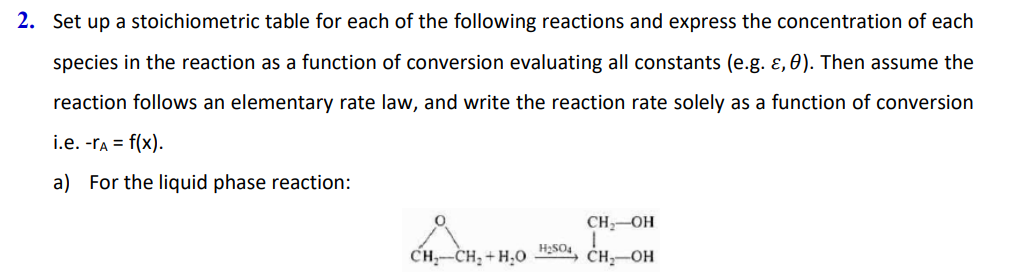 (a)
(a)
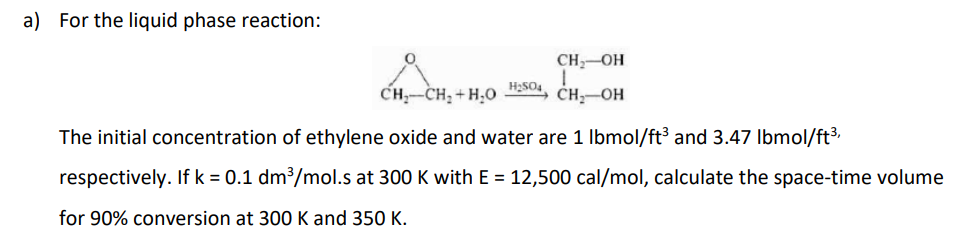
2. Set up a stoichiometric table for each of the following reactions and express the concentration of each species in the reaction as a function of conversion evaluating all constants (e.g. ,0). Then assume the reaction follows an elementary rate law, and write the reaction rate solely as a function of conversion i.e. -ra = f(x). a) For the liquid phase reaction: CH-OH CH-CH, H,O H2504 CH-OH a) For the liquid phase reaction: CH-OH CH --CH+H,0 H2SO4, CH-OH The initial concentration of ethylene oxide and water are 1 lbmol/ft? and 3.47 lbmol/ft3, respectively. If k = 0.1 dm3/mol.s at 300 K with E = 12,500 cal/mol, calculate the space-time volume for 90% conversion at 300 K and 350 K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


