Answered step by step
Verified Expert Solution
Question
1 Approved Answer
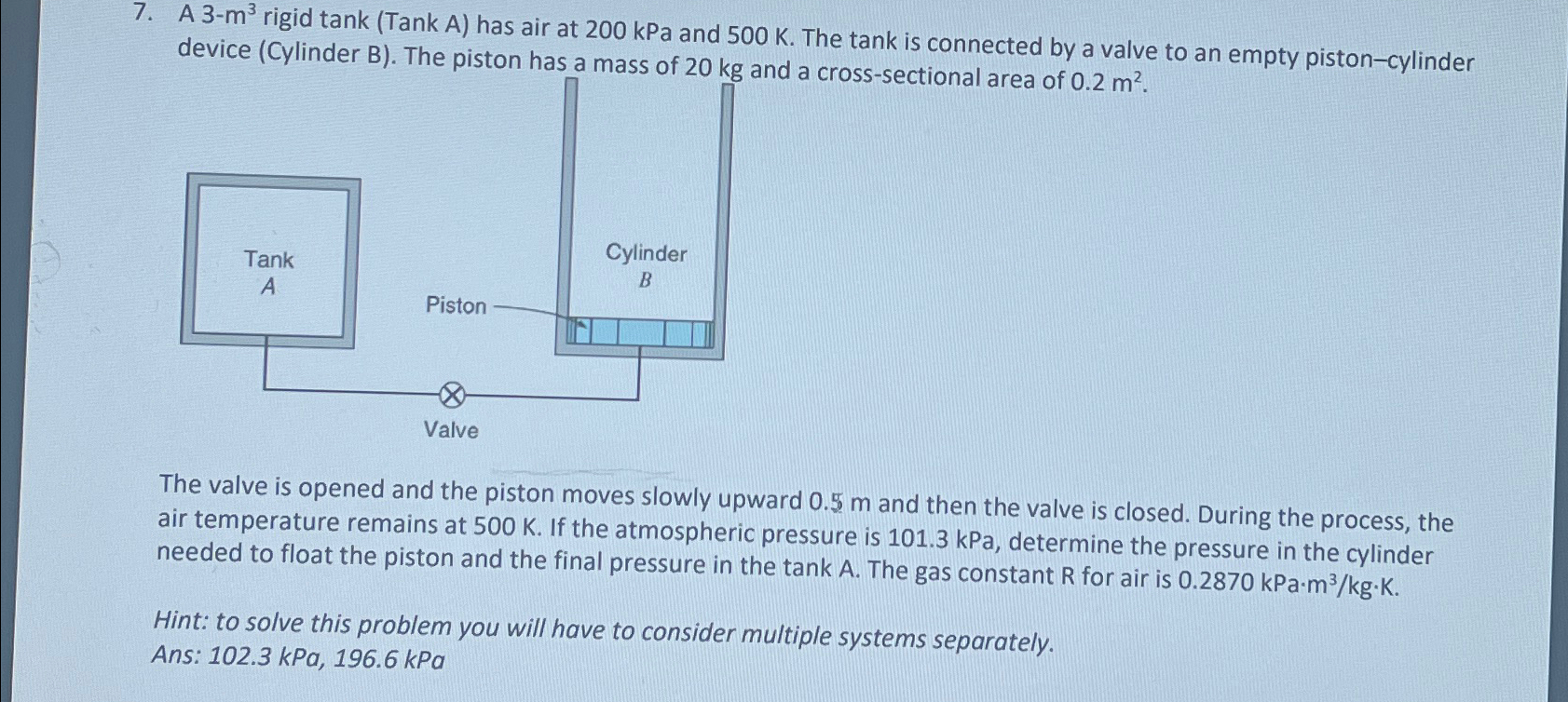
A 3 - m 3 rigid tank ( Tank A ) has air at 2 0 0 kPa and 5 0 0 K . The
A rigid tank Tank A has air at kPa and The tank is connected by a valve to an empty pistoncylinder device Cylinder B The piston has a mass of and a crosssectional area of
The valve is opened and the piston moves slowly upward and then the valve is closed. During the process, the air temperature remains at If the atmospheric pressure is kPa, determine the pressure in the cylinder needed to float the piston and the final pressure in the tank A The gas constant R for air is kPa
Hint: to solve this problem you will have to consider multiple systems separately.
Ans: kPa,kPa
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


