Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 300 cfm stream of an airSO2 mixture containing 16.2% SO2 by volume at 68 F and 1.0 atm is to be scrubbed with water
A 300 cfm stream of an air‐SO2 mixture containing 16.2% SO2 by volume at 68 °F and 1.0 atm is to be scrubbed with water in a countercurrent packed tower for the purpose of recovering 95% of the SO2. The tower operates at constant temperature.
- Draw the equilibrium line
- Determine the inlet and outlet mole fractions in water and air
- Draw the minimum operating line
- Calculate the minimum water requirement.
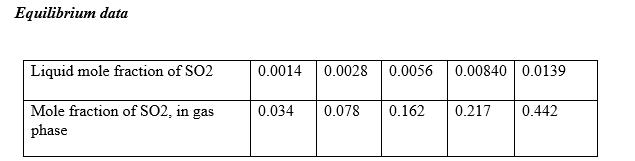
Equilibrium data Liquid mole fraction of SO2 0.0014 0.0028 0.0056 0.00840 0.0139 Mole fraction of SO2, in gas phase 0.034 0.078 0.162 0.217 0.442
Step by Step Solution
★★★★★
3.45 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1
volumetric flowrate of AirSO2 mixture 300 ft3min Here Air is carrier gas SO2 is the solute transferred from Air to Water Water is the solvent We have to convert the volumetric flowrate into molar flow...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


