Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 38-kg copper block initially at 140C is dropped into an insulated tank that contains 90 L of water at 10C. Determine the final
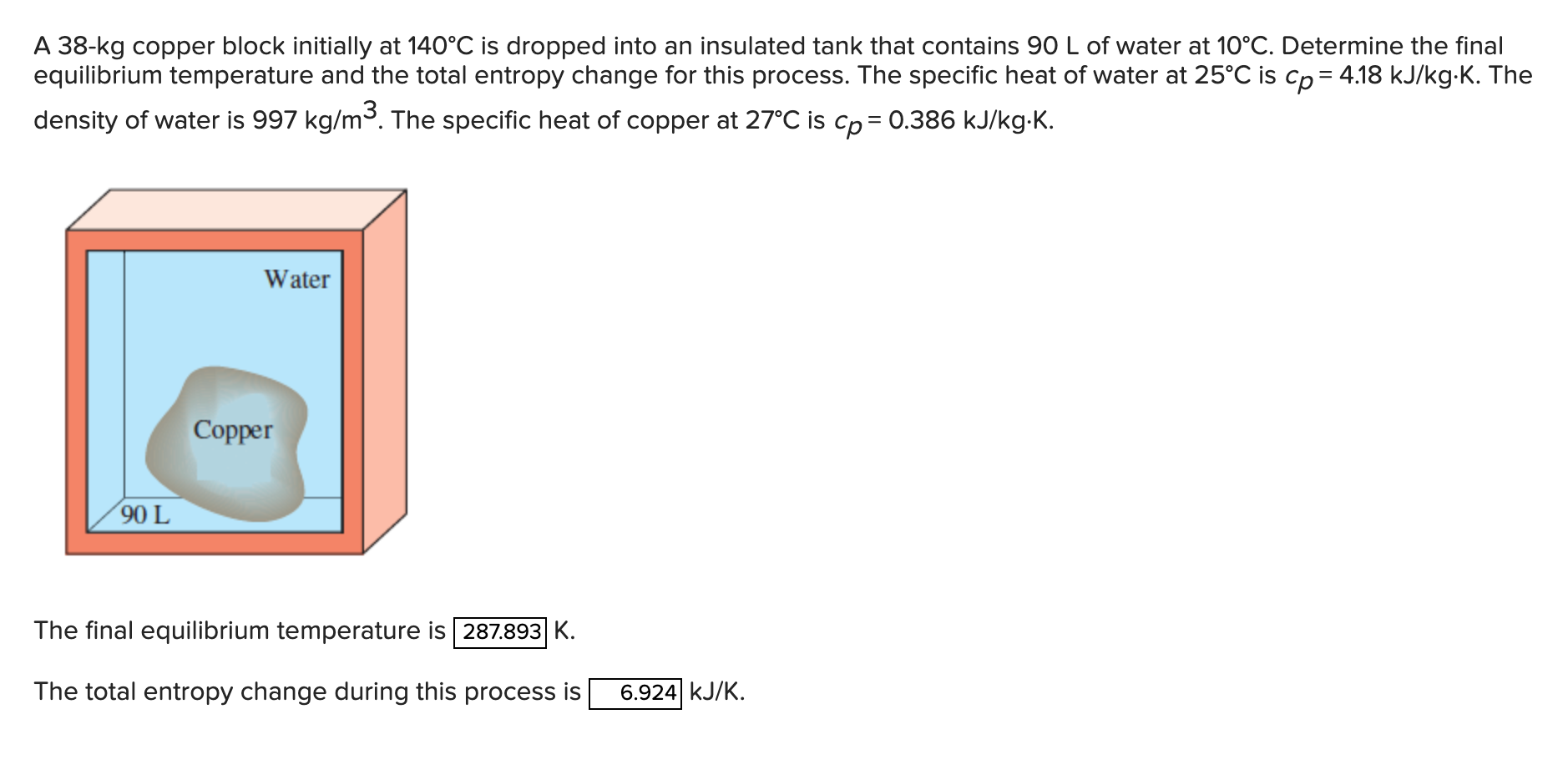
A 38-kg copper block initially at 140C is dropped into an insulated tank that contains 90 L of water at 10C. Determine the final equilibrium temperature and the total entropy change for this process. The specific heat of water at 25C is cp = 4.18 kJ/kg.K. The density of water is 997 kg/m. The specific heat of copper at 27C is cp = 0.386 kJ/kgK. 90 L Copper Water The final equilibrium temperature is 287.893 K. The total entropy change during this process is 6.924 KJ/K.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


