Answered step by step
Verified Expert Solution
Question
1 Approved Answer
a) A stream contains a mixture of 40% normal heptane (by mol) and 60% toluene. Calculate the bubble and dew pressure of the mixture
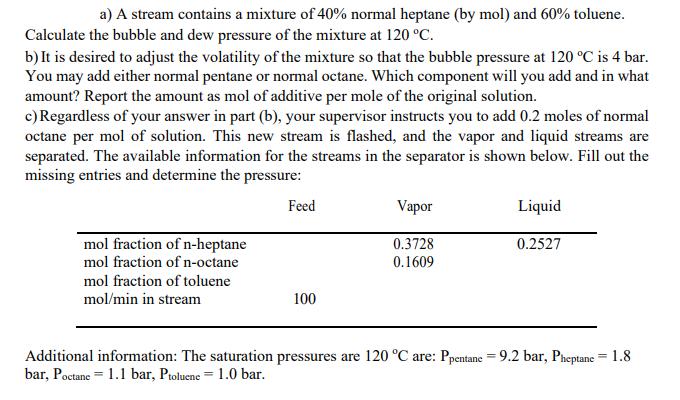
a) A stream contains a mixture of 40% normal heptane (by mol) and 60% toluene. Calculate the bubble and dew pressure of the mixture at 120 C. b) It is desired to adjust the volatility of the mixture so that the bubble pressure at 120 C is 4 bar. You may add either normal pentane or normal octane. Which component will you add and in what amount? Report the amount as mol of additive per mole of the original solution. c) Regardless of your answer in part (b), your supervisor instructs you to add 0.2 moles of normal octane per mol of solution. This new stream is flashed, and the vapor and liquid streams are separated. The available information for the streams in the separator is shown below. Fill out the missing entries and determine the pressure: Feed Vapor Liquid mol fraction of n-heptane 0.3728 0.2527 mol fraction of n-octane 0.1609 mol fraction of toluene mol/min in stream 100 Additional information: The saturation pressures are 120 C are: Ppentane = 9.2 bar, Pheptane = 1.8 bar, Poctane 1.1 bar, Ptoluene 1.0 bar. =
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
a To calculate the bubble and dew pressures of the mixture at 120C we need to use Raoults Law which ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


