Answered step by step
Verified Expert Solution
Question
1 Approved Answer
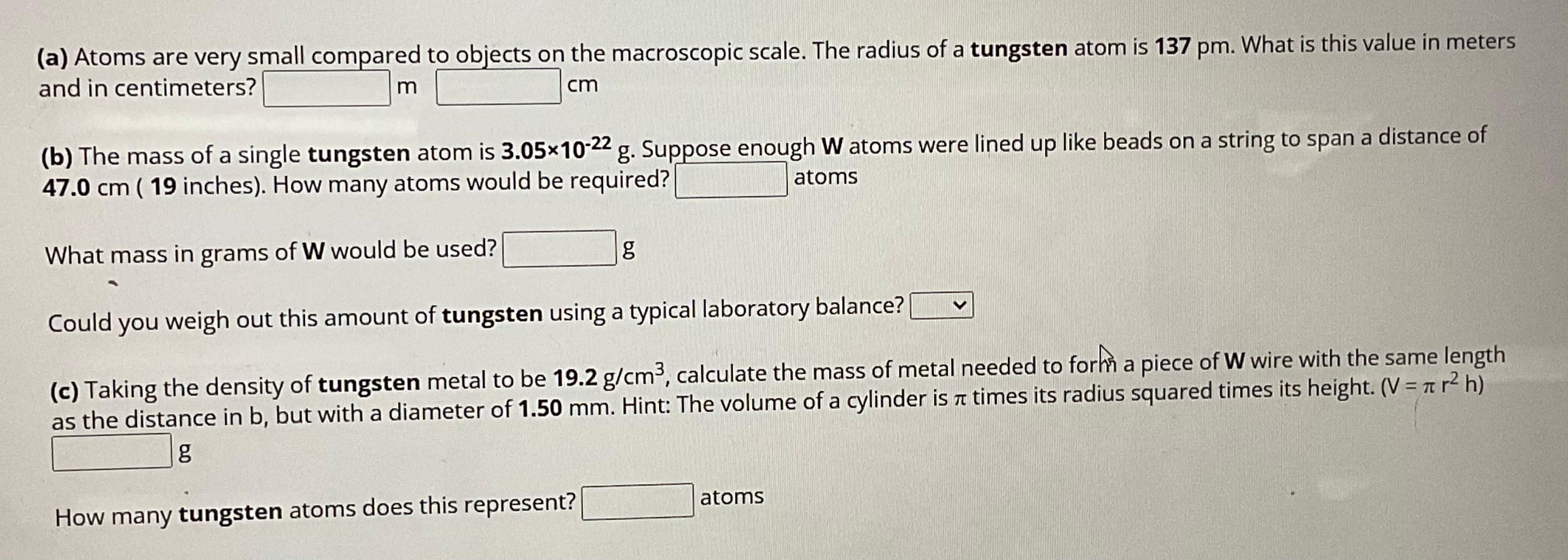
( a ) Atoms are very small compared to objects on the macroscopic scale. The radius of a tungsten atom is 1 3 7 p
a Atoms are very small compared to objects on the macroscopic scale. The radius of a tungsten atom is What is this value in meters and in centimeters?
b The mass of a single tungsten atom is Suppose enough atoms were lined up like beads on a string to span a distance of inches How many atoms would be required? atoms
What mass in grams of would be used?
g
Could you weigh out this amount of tungsten using a typical laboratory balance?
c Taking the density of tungsten metal to be calculate the mass of metal needed to forhn a piece of W wire with the same length as the distance in but with a diameter of Hint: The volume of a cylinder is times its radius squared times its height. Vg
How many tungsten atoms does this represent? atoms
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


