Answered step by step
Verified Expert Solution
Question
1 Approved Answer
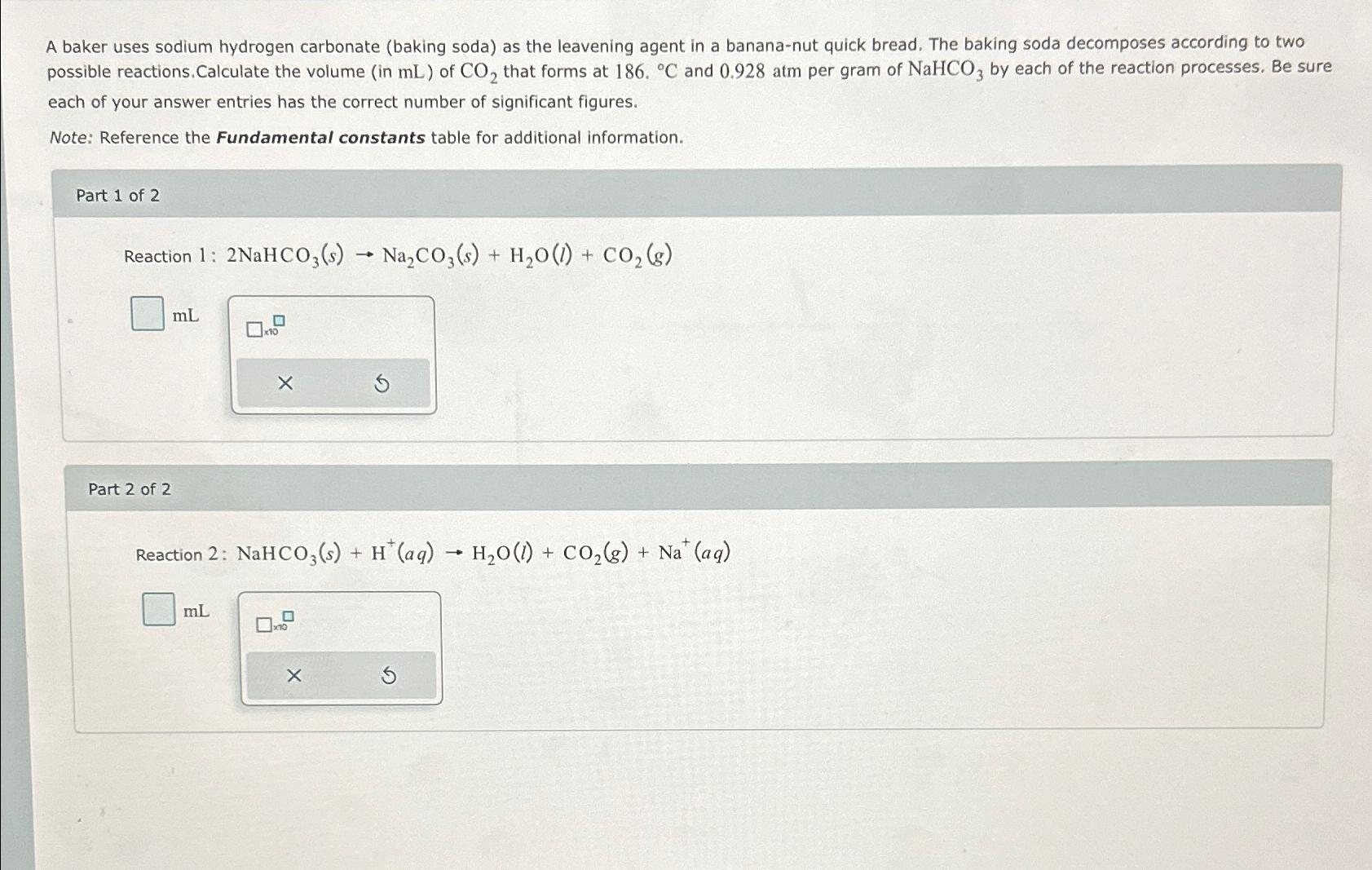
A baker uses sodium hydrogen carbonate ( baking soda ) as the leavening agent in a banana - nut quick bread. The baking soda decomposes
A baker uses sodium hydrogen carbonate baking soda as the leavening agent in a banananut quick bread. The baking soda decomposes according to two possible reactions. Calculate the volume in of that forms at and atm per gram of by each of the reaction processes. Be sure each of your answer entries has the correct number of significant figures.
Note: Reference the Fundamental constants table for additional information.
Part of
Reaction :
Part of
Reaction :
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


