Answered step by step
Verified Expert Solution
Question
1 Approved Answer
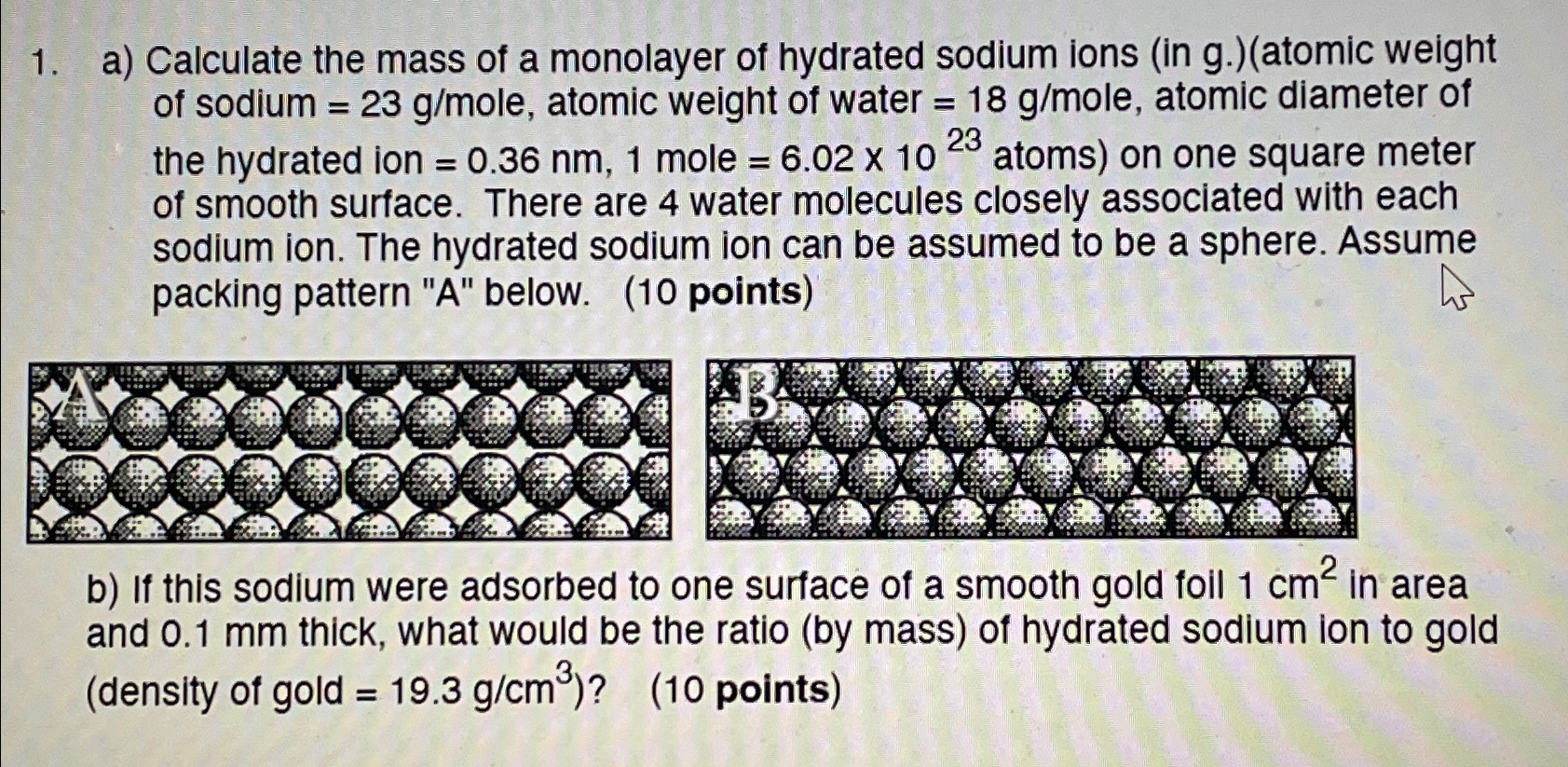
a ) Calculate the mass of a monolayer of hydrated sodium ions ( in g . ) ( atomic weight of sodium = 2 3
a Calculate the mass of a monolayer of hydrated sodium ions in gatomic weight of sodium ole, atomic weight of water ole, atomic diameter of the hydrated ion mole atoms on one square meter of smooth surface. There are water molecules closely associated with each sodium ion. The hydrated sodium ion can be assumed to be a sphere. Assume packing pattern below. points
b If this sodium were adsorbed to one surface of a smooth gold foil in area and thick, what would be the ratio by mass of hydrated sodium ion to gold density of gold points
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


