Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chememicas engineer is studying the two reactions shown in the table below. In each Case. She fills al reaction vessel with some mixture of
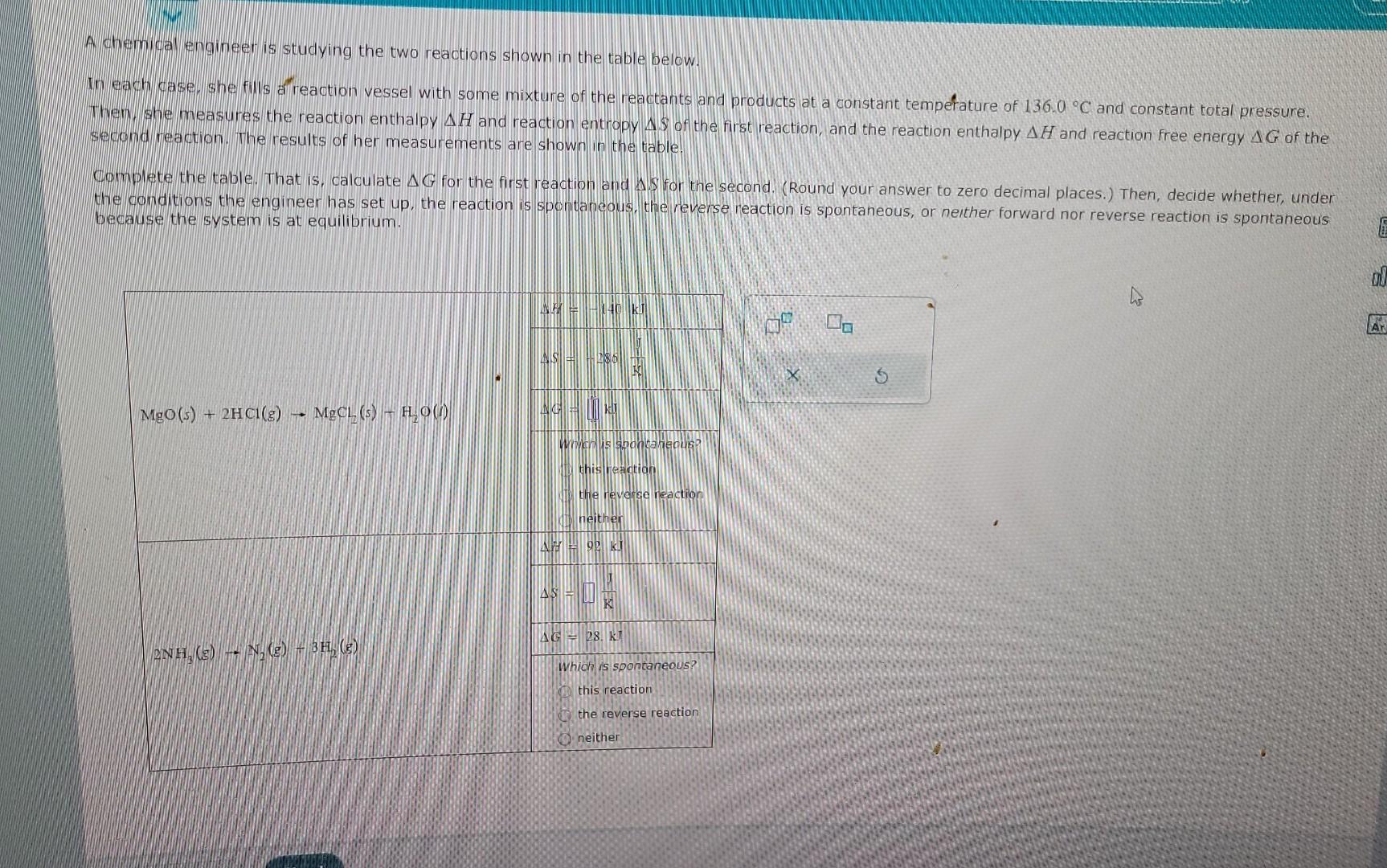
A chememicas engineer is studying the two reactions shown in the table below. In each Case. She fills al reaction vessel with some mixture of the reactants and products at a constant tempefature of 136.0C and constant total pressure. Thene, she measures the reaction enthalpy H and reaction entropy $ of the first reaction, and the reaction enthalpy H and reaction free energy G of the sectond reaction. The results of her measurements are shown in the table. Gomplete the table. That is, calculate G for the first reaction and .S for the second. (Round your answer to zero decimal places.) Then, decide whether, under the conditions the engineer has set up, the reaction is spontanepus, the reverse reaction is spontaheous, or neither forward nor reverse reaction is spontaneous because the system is at equilibrium
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


