Question
A chemical company is producing acetylene (C2H2). The acetylene is produced by reacting methane (CH4) and oxygen (O2) according to the given reaction equations. Prior
A chemical company is producing acetylene (C2H2). The acetylene is produced by reacting methane (CH4) and oxygen (O2) according to the given reaction equations. Prior to the reaction, both CH4 and O2 at 25oC are mixed (M) and heated (H) to 600oC. The fed CH4 is in excess by the range of (100 - 200)% excess. The heated reactants enter the Reactor (R), the fed O2 is completely consumed by the incomplete combustion reaction (reaction 1) and the yield of C2H2 is 0.25. The product stream leaves the reactor at a temperature range of (600-700)oC and is immediately quenched or cooled in the quenching tower (Q) to 40oC by cooling liquid water. The cooling liquid water enters at 14oC and leaves as steam at pressure of 5 bar. The water vapor from the incomplete combustion reaction is merged with the exit cooling water system during the quenching. The cooled products enter Separator 1, where CO and H2 were discarded while CH4 and C2H2 enter Separator 2. From Separator 2, CH4 is recycled to the mixer while the C2H2 is collected as the final product.
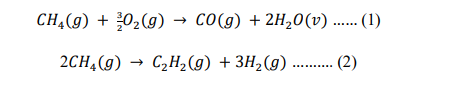
a) Draw the process flow diagram and completely label each stream and unit operation.
b) Report energy balance calculation of the reactor. A one (1) percentage of excess CH4 and a one (1) process condition (reactor outlet stream temperature) should be selected based on the given ranges. All assumptions in the calculation should be stated.
PLEASE ANSWER ALL QUESTION CORRECTLY. THANKYOU !
CH4(g)+23O2(g)CO(g)+2H2O(v) 2CH4(g)C2H2(g)+3H2(g)..(2)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


