Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A chromophore is pipetted according to the scheme in table 1 to establish a calibration curve for measuring the concentration of the chromophore in an

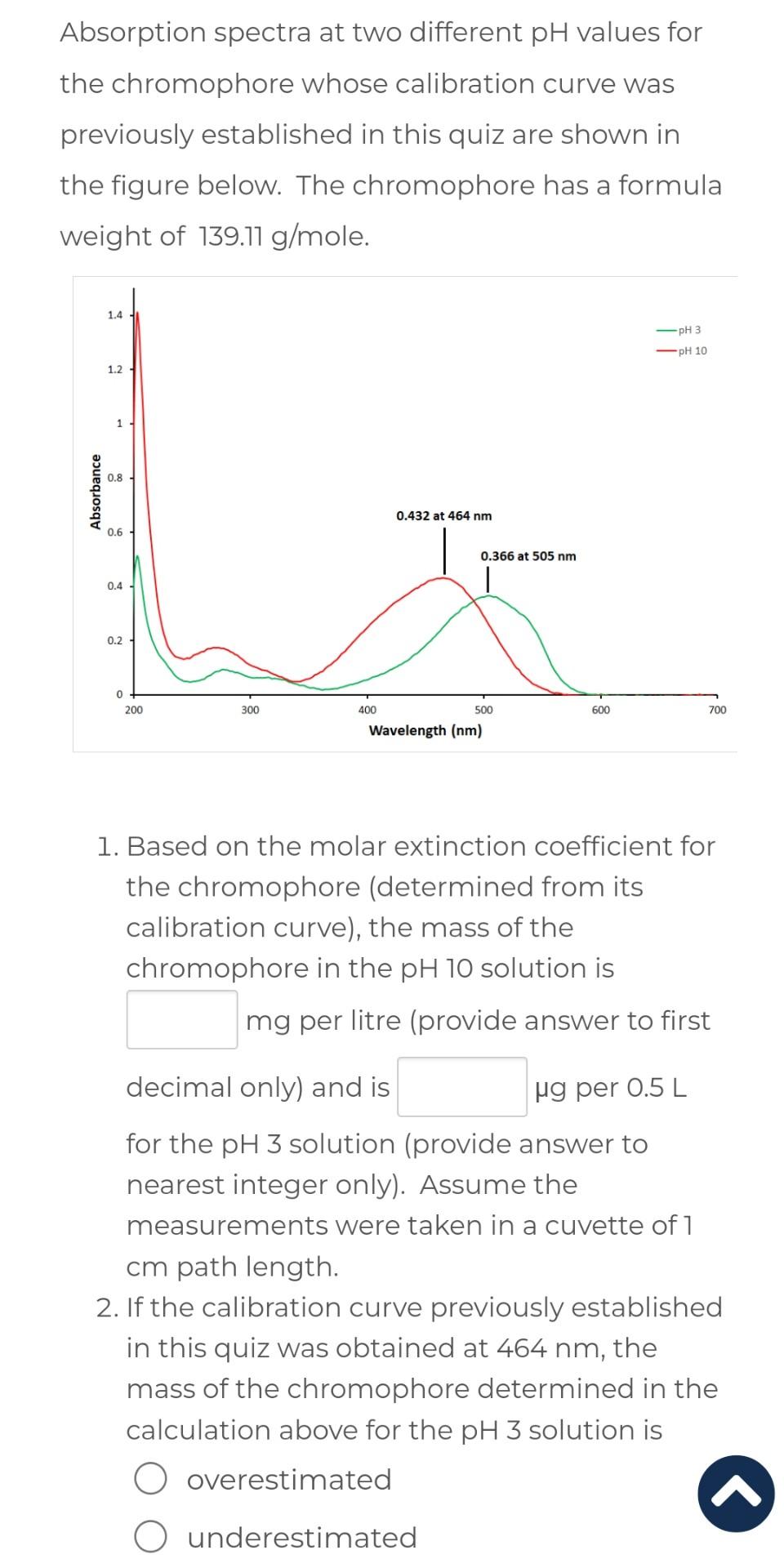
A chromophore is pipetted according to the scheme in table 1 to establish a calibration curve for measuring the concentration of the chromophore in an unknown solution. The absorbance measurements are obtained at the wavelength of maximum absorption for the chromophore, for three independently pipetted test tubes at each concentration level. B, reference blank solution. Table 1: Calibration curve data for chromophore. 1. Construct a reference-corrected calibration curve for the chromophore and upload the saved image file for the graph in the Atto window provided (NB: Please view the video showing how to upload images to the Moodle Atto window prior to attempting this quiz). Your calibration curve must plot the average reference-corrected absorbance measurements the standard deviation (as error bars) as a function of the standard chromophore concentration. 2. What does the standard deviation measure, statistically? Absorption spectra at two different pH values for the chromophore whose calibration curve was previously established in this quiz are shown in the figure below. The chromophore has a formula weight of 139.11g/mole. 1. Based on the molar extinction coefficient for the chromophore (determined from its calibration curve), the mass of the chromophore in the pH10 solution is mg per litre (provide answer to first decimal only) and is g per 0.5L for the pH3 solution (provide answer to nearest integer only). Assume the measurements were taken in a cuvette of 1 cm path length. 2. If the calibration curve previously established in this quiz was obtained at 464nm, the mass of the chromophore determined in the calculation above for the pH3 solution is overestimated underestimated
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


