A cloud is observed to commence formation at a temperature of-61 C and continues to cool due to emission of infrared radiation. a) Determine
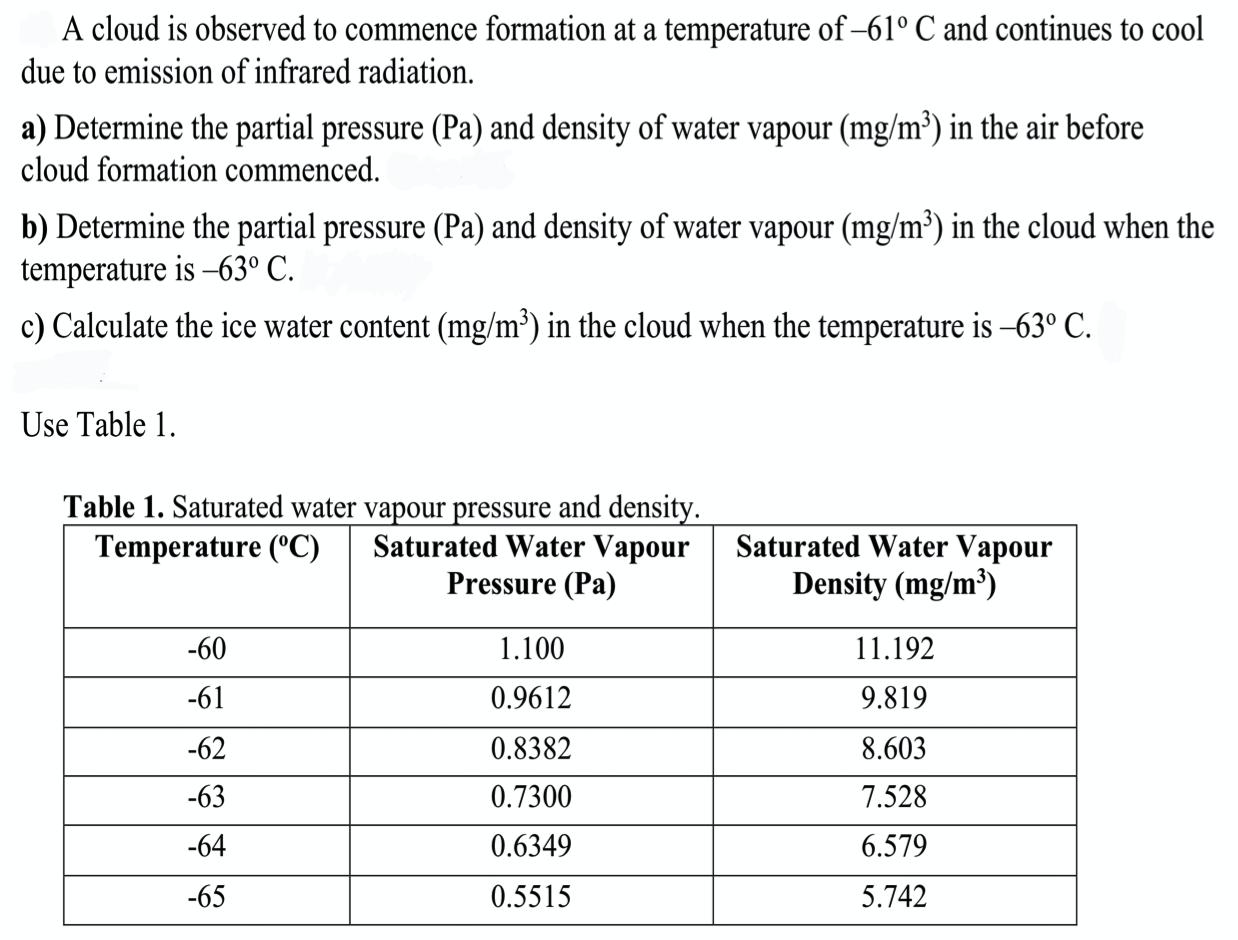
A cloud is observed to commence formation at a temperature of-61 C and continues to cool due to emission of infrared radiation. a) Determine the partial pressure (Pa) and density of water vapour (mg/m) in the air before cloud formation commenced. b) Determine the partial pressure (Pa) and density of water vapour (mg/m) in the cloud when the temperature is -63 C. c) Calculate the ice water content (mg/m) in the cloud when the temperature is 63 C. Use Table 1. Table 1. Saturated water vapour pressure and density. Temperature (C) Saturated Water Vapour Pressure (Pa) -60 -61 -62 -63 -64 -65 1.100 0.9612 0.8382 0.7300 0.6349 0.5515 Saturated Water Vapour Density (mg/m) 11.192 9.819 8.603 7.528 6.579 5.742
Step by Step Solution
3.47 Rating (170 Votes )
There are 3 Steps involved in it
Step: 1
Answer Aus mer Temp 61C a Partial pressure ...
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


