Question
A compressor is being used to compress air reversibly from 1 bar and 293 K to 15 bar. The compression can be assumed to
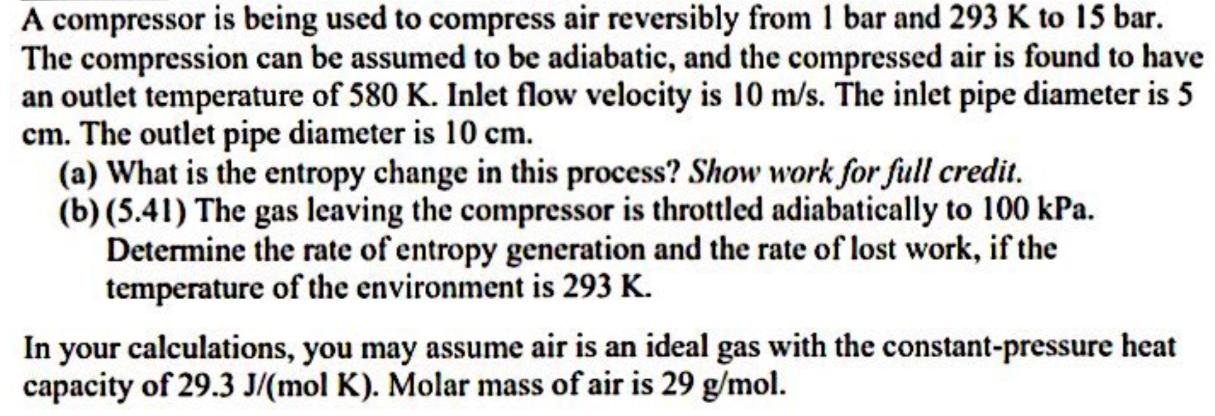
A compressor is being used to compress air reversibly from 1 bar and 293 K to 15 bar. The compression can be assumed to be adiabatic, and the compressed air is found to have an outlet temperature of 580 K. Inlet flow velocity is 10 m/s. The inlet pipe diameter is 5 cm. The outlet pipe diameter is 10 cm. (a) What is the entropy change in this process? Show work for full credit. (b) (5.41) The gas leaving the compressor is throttled adiabatically to 100 kPa. Determine the rate of entropy generation and the rate of lost work, if the temperature of the environment is 293 K. In your calculations, you may assume air is an ideal gas with the constant-pressure heat capacity of 29.3 J/(mol K). Molar mass of air is 29 g/mol.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Biochemical And Engineering Thermodynamics
Authors: Stanley I. Sandler
5th Edition
047050479X, 978-0470504796
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



