Question
A copper, Cu(s), electrode is immersed in a solution that is 1.00 M in ammonia, NH3, and 1.00 M in tetraamminecopper(II). [Cu(NH3)4]2+ If a
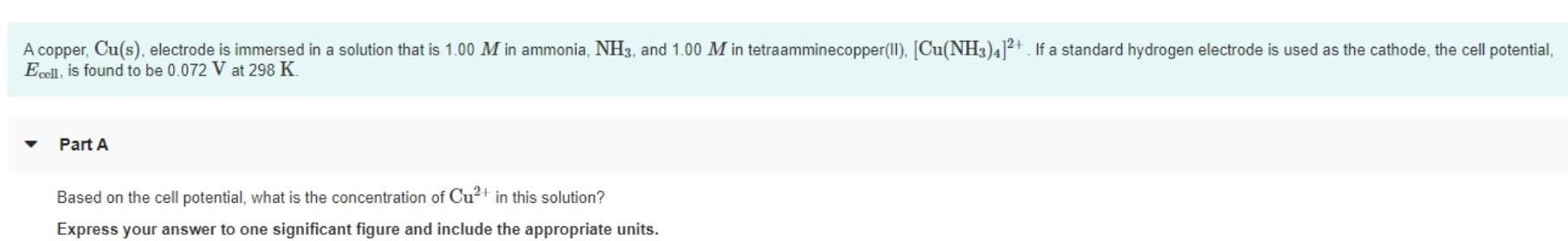
A copper, Cu(s), electrode is immersed in a solution that is 1.00 M in ammonia, NH3, and 1.00 M in tetraamminecopper(II). [Cu(NH3)4]2+ If a standard hydrogen electrode is used as the cathode, the cell potential, Ecell, is found to be 0.072 V at 298 K. Part A Based on the cell potential, what is the concentration of Cu+ in this solution? Express your answer to one significant figure and include the appropriate units.
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



