Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A cylinder with a movable piston contains 5.00 liters of a gas at 30C and 5.00 bar. The piston is slowly moved to compress the
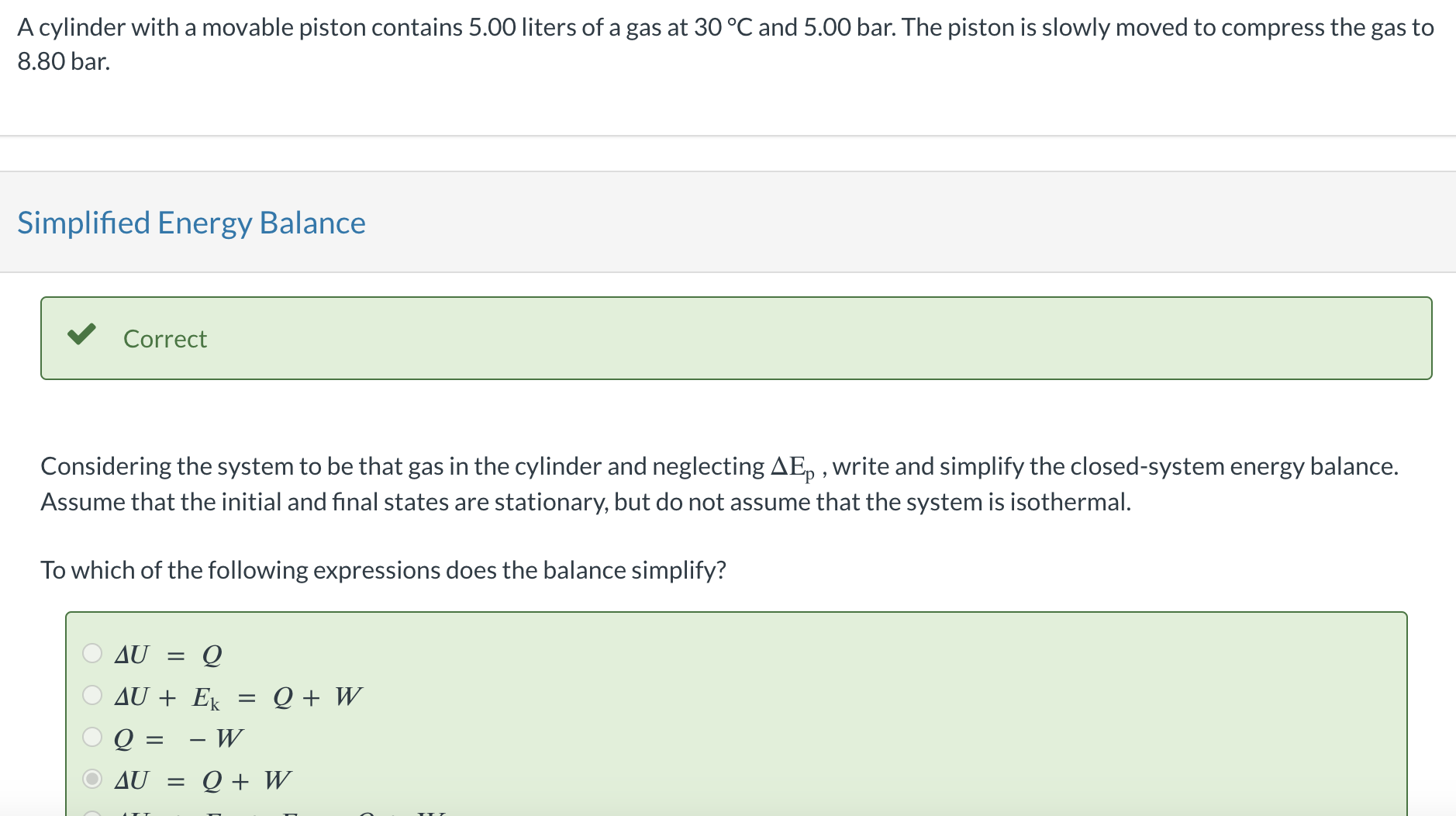
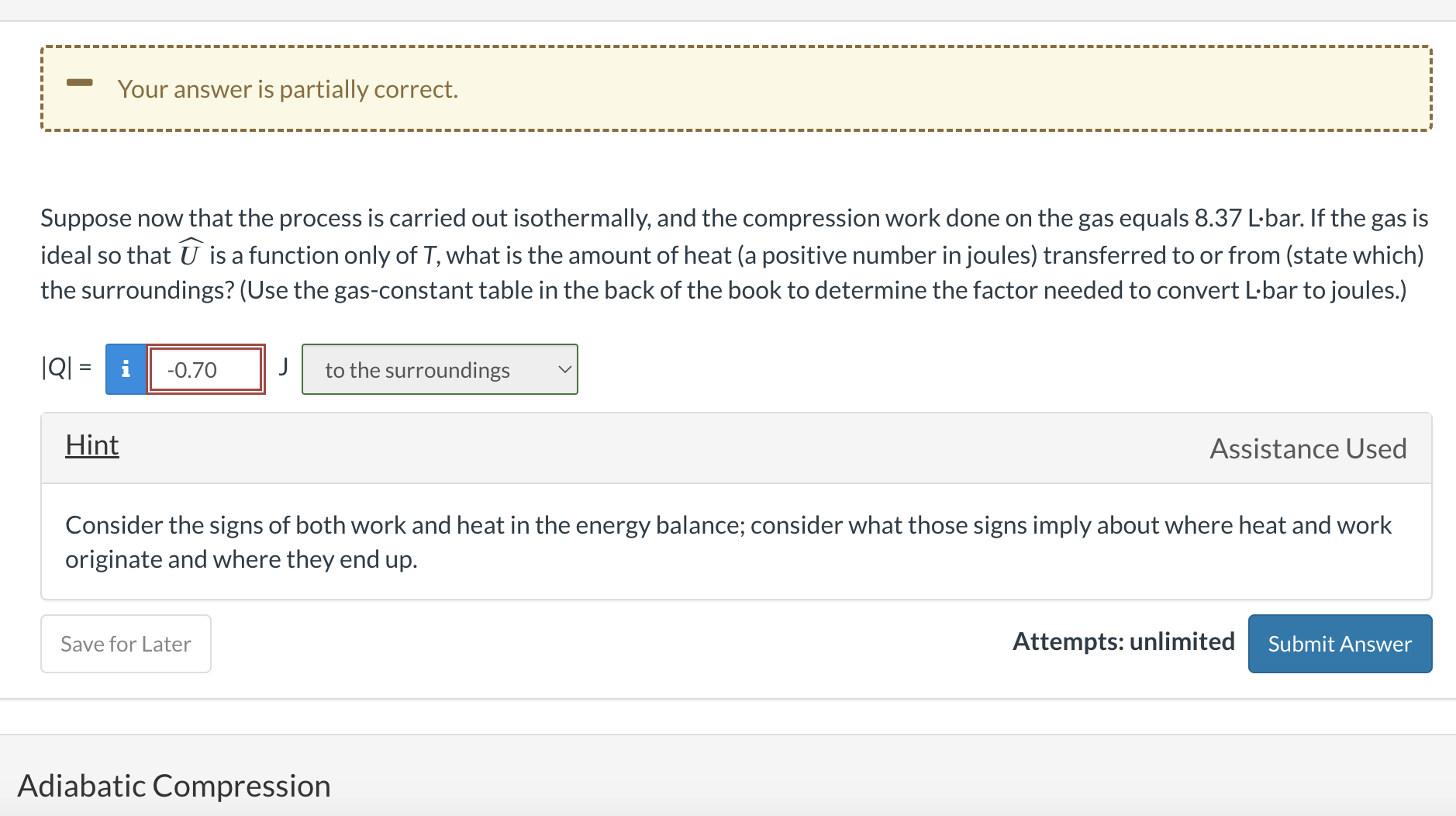 A cylinder with a movable piston contains 5.00 liters of a gas at 30C and 5.00 bar. The piston is slowly moved to compress the gas to 8.80 bar. Simplified Energy Balance Correct Considering the system to be that gas in the cylinder and neglecting Ep, write and simplify the closed-system energy balance. Assume that the initial and final states are stationary, but do not assume that the system is isothermal. To which of the following expressions does the balance simplify? U=QU+Ek=Q+WQ=WU=Q+W Suppose now that the process is carried out isothermally, and the compression work done on the gas equals 8.37L bar. If the gas is ideal so that U is a function only of T, what is the amount of heat (a positive number in joules) transferred to or from (state which) the surroundings? (Use the gas-constant table in the back of the book to determine the factor needed to convert Lbar to joules.) Q=J Hint Assistance Used Consider the signs of both work and heat in the energy balance; consider what those signs imply about where heat and work originate and where they end up. Attempts: unlimited Adiabatic Compression
A cylinder with a movable piston contains 5.00 liters of a gas at 30C and 5.00 bar. The piston is slowly moved to compress the gas to 8.80 bar. Simplified Energy Balance Correct Considering the system to be that gas in the cylinder and neglecting Ep, write and simplify the closed-system energy balance. Assume that the initial and final states are stationary, but do not assume that the system is isothermal. To which of the following expressions does the balance simplify? U=QU+Ek=Q+WQ=WU=Q+W Suppose now that the process is carried out isothermally, and the compression work done on the gas equals 8.37L bar. If the gas is ideal so that U is a function only of T, what is the amount of heat (a positive number in joules) transferred to or from (state which) the surroundings? (Use the gas-constant table in the back of the book to determine the factor needed to convert Lbar to joules.) Q=J Hint Assistance Used Consider the signs of both work and heat in the energy balance; consider what those signs imply about where heat and work originate and where they end up. Attempts: unlimited Adiabatic Compression Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


