Question
A deep sea diver breathes a mixture of 21% O2 and 79% He, which is at the local water pressure. The divers body contains
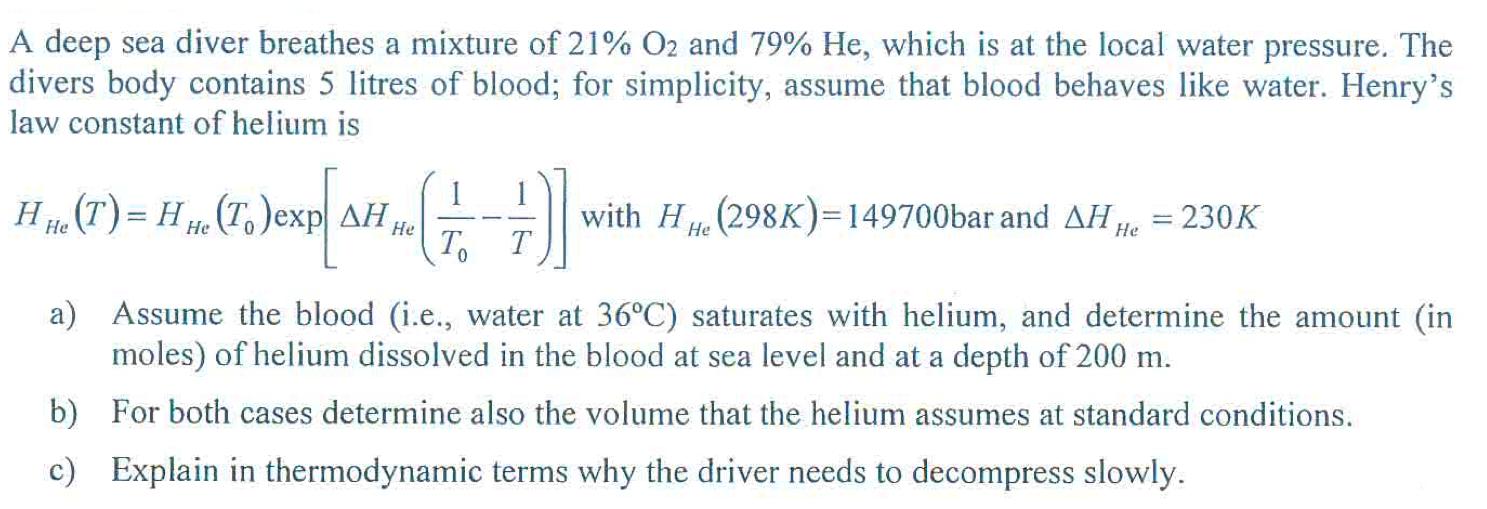
A deep sea diver breathes a mixture of 21% O2 and 79% He, which is at the local water pressure. The divers body contains 5 litres of blood; for simplicity, assume that blood behaves like water. Henry's law constant of helium is H He (T) = H He (To)exp AH He 1 To })] with HH (298K)=149700bar and AHH He T = 230K = a) Assume the blood (i.e., water at 36C) saturates with helium, and determine the amount (in moles) of helium dissolved in the blood at sea level and at a depth of 200 m. b) For both cases determine also the volume that the helium assumes at standard conditions. c) Explain in thermodynamic terms why the driver needs to decompress slowly.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a At sea level 0 m the partial pressure of He is 0 bar So no He dissolves in the blo...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Modern Operating Systems
Authors: Andrew S. Tanenbaum, Herbert Bos
4th edition
013359162X, 978-0133591620
Students also viewed these General Management questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



