Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A differential reactor is used to evaluate the kinetics of the vapor-phase reaction A+2BC+D The table below contains the measured rate of formation of product
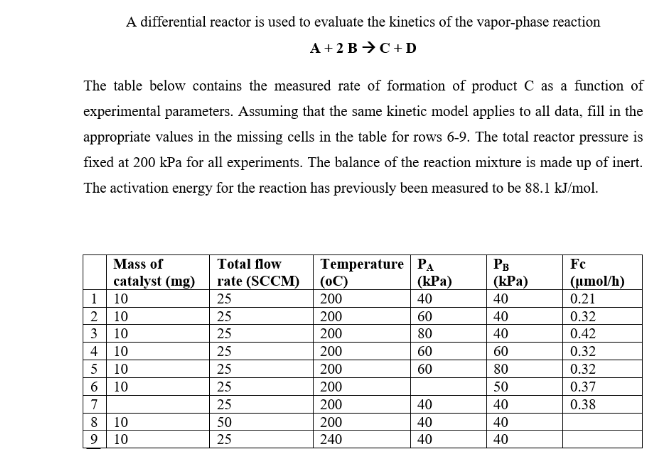 A differential reactor is used to evaluate the kinetics of the vapor-phase reaction A+2BC+D The table below contains the measured rate of formation of product C as a function of experimental parameters. Assuming that the same kinetic model applies to all data, fill in the appropriate values in the missing cells in the table for rows 6-9. The total reactor pressure is fixed at 200kPa for all experiments. The balance of the reaction mixture is made up of inert. The activation energy for the reaction has previously been measured to be 88.1kJ/mol
A differential reactor is used to evaluate the kinetics of the vapor-phase reaction A+2BC+D The table below contains the measured rate of formation of product C as a function of experimental parameters. Assuming that the same kinetic model applies to all data, fill in the appropriate values in the missing cells in the table for rows 6-9. The total reactor pressure is fixed at 200kPa for all experiments. The balance of the reaction mixture is made up of inert. The activation energy for the reaction has previously been measured to be 88.1kJ/mol Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


