Question
A dimerization reaction of 2 R molecules occurs on a catalyst surface to form a product P. The reaction proceeds through the following mechanism:
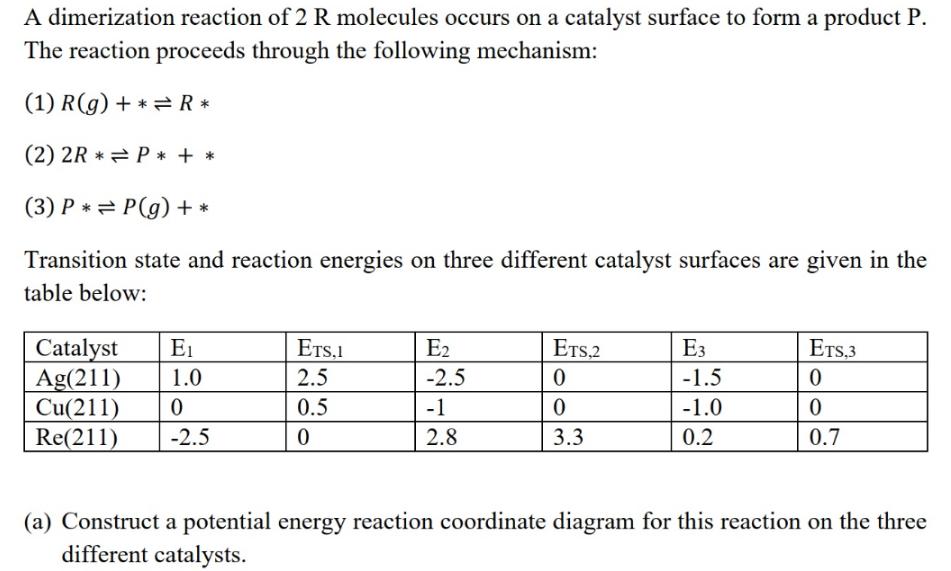
A dimerization reaction of 2 R molecules occurs on a catalyst surface to form a product P. The reaction proceeds through the following mechanism: (1) R(g) +* R* (2) 2R* P*+ * (3) P = P(g) + * Transition state and reaction energies on three different catalyst surfaces are given in the table below: Catalyst E Ag(211) 1.0 Cu(211) 0 Re(211) -2.5 ETS.1 2.5 0.5 0 E -2.5 -1 2.8 ETS.2 0 0 3.3 E3 -1.5 -1.0 0.2 ETS,3 0 0 0.7 (a) Construct a potential energy reaction coordinate diagram for this reaction on the three different catalysts.
Step by Step Solution
3.37 Rating (141 Votes )
There are 3 Steps involved in it
Step: 1
These diagrams illus...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry A Molecular Approach
Authors: Nivaldo Tro
5th Edition
0134874374, 978-0134874371
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



