Which gas sample has the greatest pressure? Assume that all the samples are at the same temperature.
Question:
Which gas sample has the greatest pressure? Assume that all the samples are at the same temperature. Explain.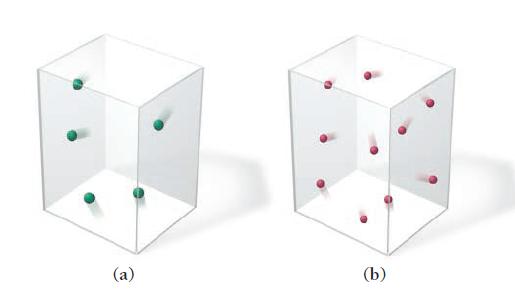
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider a 1.0-L sample of helium gas and a 1.0-L sample of argon gas, both at room temperature and atmospheric pressure. a. Do the atoms in the helium sample have the same average kinetic energy as...
-
Consider two gases, A and B, each in a 1.0- L container with both gases at the same temperature and pressure. The mass of gas A in the container is 0.34 g, and the mass of gas B in the container is...
-
Which gas sample has the greatest volume at STP? a) 10.0 g Ar b) 10.0 g Kr c) 10.0 g Xe d) None of the above (They all have the same volume.)
-
Consider a tank containing a liquid, and the rate of change of the liquid's height (h) with respect to time (t) is proportional to the difference between the current height and a reference height....
-
The Sorrento Hotel is a four-star hotel located in downtown Seattle. The hotels operations vice president would like to replace the hotels antiquated computer terminals at the registration desk with...
-
The following is an extract from a newspaper article in the financial press. Investors are becoming increasingly dissatisfied with annual reports because these are backward-looking, historical...
-
Prove that the weights in Eq. (11.18) add up to one. (Hint: Use the geometric series.)
-
Each autumn, as a hobby, Suzanne De Angelo weaves cotton placemats to sell at a local crafts shop. The mats sell for $20 per set of four. The shop charges a 10% commission and remits the net proceeds...
-
Stuart Chairs, Inc. makes two types of chairs. Model Diamond is a high-end product designed for professional offices. Model Gold is an economical product designed for family use. Jane Silva, the...
-
Haidilao is a popular hotpot chain in Singapore. The restaurant is famous for its good quality food and superb services. During peak hours, crowds of customers need to sit outside the store, waiting...
-
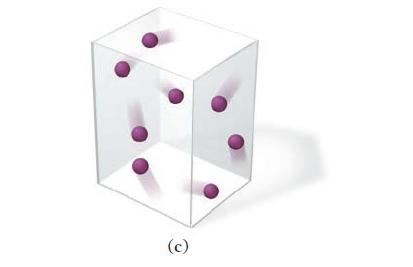
This picture represents a sample of gas at a pressure of 1 atm, a volume of 1 L, and a temperature of 25 C. Draw a similar picture showing what would happen to the sample if the volume were reduced...
-
A wine-dispensing system uses argon canisters to pressurize and preserve wine in the bottle. An argon canister for the system has a volume of 55.0 mL and contains 26.0 g of argon. Assuming ideal gas...
-
Use the following random sample of suitcase prices to construct a 90% confidence interval for the average suitcase price. $285, 110, 495, 119, 450, 125, 250, 320
-
Q.9 Prepare a cash flow statement using the indirect method based on the following information: - Net Income: $150,000 - Depreciation Expense: $20,000 - Increase in Accounts Receivable: $10,000...
-
3.11 (a) Find the order of the elements 2, 7, 10 and 12 in F17. (b) Find the order of the elements a, a, a + 1 and a3 + 1 in F16, where a is a root of 1+x+x4.
-
You have been recently hired to lead a Project to relocate your main Distribution Centre (DC) from Calgary, Alberta to St. John's, Newfoundland. As the Project Manager, try to complete a project plan...
-
Males Mean: 69.6 Standard Deviation: 11.3 For males, find P90, which is the pulse rate separating the bottom 90% from the top 10%.
-
Statistics Assignments Using Excel Assignment #4: Measures of Variability Part I Below are ACT composite scores from 20 randomly selected college students. 15 33 20 25 21 24 17 16 20 25 26 21 21 17...
-
Construct radial hardness profiles for the following: (a) A 50-mm (2-in.) diameter cylindrical specimen of an 8640 steel alloy that has been quenched in moderately agitated oil (b) A 75-mm (3-in.)...
-
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke =...
-
Using Castiglianos theorem and determine the horizontal deflection at C. EI is constant. There is a pin at A. Assume C is a roller and B is a fixed joint. 400 lb/ft 6 ft 10 ft 45
-
Use the method of virtual work and determine the horizontal deflection at C. EI is constant. There is a pin at A. Assume C is a roller and B is a fixed joint. 400 lb/ft 6 ft 10 ft 45
-
Using Castiglianos theorem and determine the horizontal deflection at C. The cross-sectional area of each member is indicated in the figure. Assume the members are pin connected at their end points....
-
Marietta Marine, Inc., has a traditional Section 401(k) plan. The actual deferral percentage (ADP) for all eligible non-highly compensated employees (non-HCEs) is 4%. What is the maximum ADP for the...
-
How long does a seller have to cure after shipping non-conforming goods?
-
Forward exchange contract designated as a fair value hedge of a foreign-currency-denominated accounts payable, strengthening $US On October 20, 2018, our company purchased from a company located in...

Study smarter with the SolutionInn App


