Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2.76 In Chapter 3, we will explore the factors that render compounds acidic or basic. Tropolone (1) is a compound that is both fairly
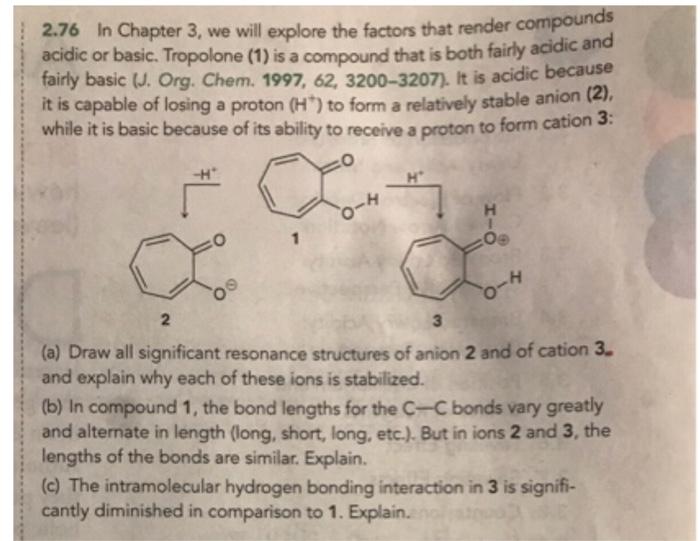
2.76 In Chapter 3, we will explore the factors that render compounds acidic or basic. Tropolone (1) is a compound that is both fairly acidic and fairly basic (J. Org. Chem. 1997, 62, 3200-3207). It is acidic because it is capable of losing a proton (H*) to form a relatively stable anion (2), while it is basic because of its ability to receive a proton to form cation 3: H 2 3. (a) Draw all significant resonance structures of anion 2 and of cation 3. and explain why each of these ions is stabilized. (b) In compound 1, the bond lengths for the C-C bonds vary greatly and alternate in length (long, short, long, etc.). But in ions 2 and 3, the lengths of the bonds are similar. Explain. (c) The intramolecular hydrogen bonding interaction in 3 is signifi- cantly diminished in comparison to 1. Explain.no
Step by Step Solution
★★★★★
3.42 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
Albion is 21 I Die Resonance stoveturers of aniona H r...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


