Answered step by step
Verified Expert Solution
Question
1 Approved Answer
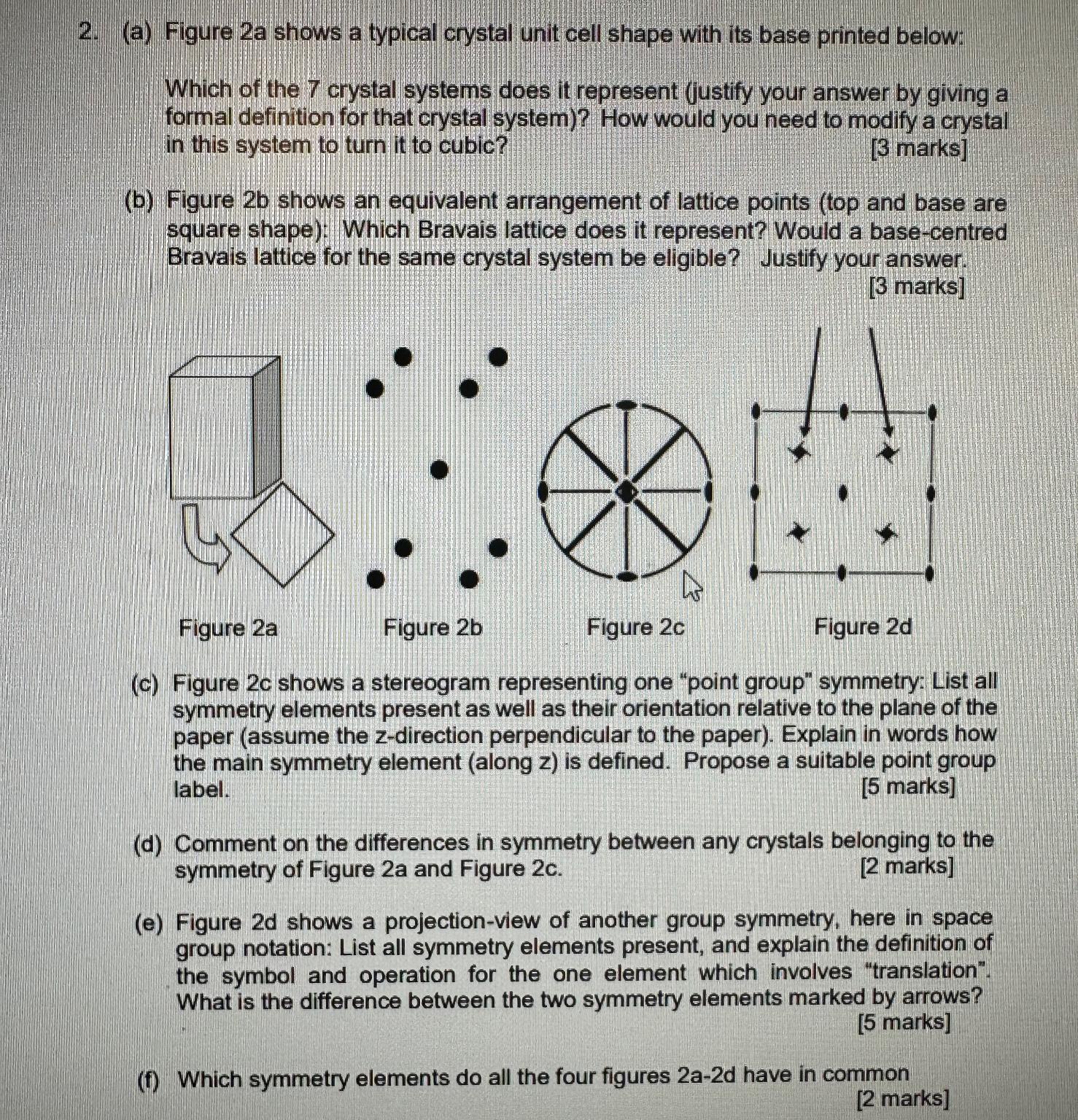
( a ) Figure 2 a shows a typical crystal unit cell shape with its base printed below: Which of the 7 crystal systems does
a Figure a shows a typical crystal unit cell shape with its base printed below:
Which of the crystal systems does it represent justify your answer by giving a formal definition for that crystal system How would you need to modify a crystal in this system to turn it to cubic?
marks
b Figure shows an equivalent arrangement of lattice points top and base are square shape: Which Bravais lattice does it represent? Would a basecentred Bravais lattice for the same crystal system be eligible? Justify your answer.
marks
c Figure shows a stereogram representing one "point group" symmetry: List all symmetry elements present as well as their orientation relative to the plane of the paper assume the zdirection perpendicular to the paper Explain in words how the main symmetry element along is defined. Propose a suitable point group label.
marks
d Comment on the differences in symmetry between any crystals belonging to the symmetry of Figure a and Figure c
marks
e Figure shows a projectionview of another group symmetry, here in space group notation: List all symmetry elements present, and explain the definition of the symbol and operation for the one element which involves "translation". What is the difference between the two symmetry elements marked by arrows?
marks
f Which symmetry elements do all the four figures have in common
marks
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


