Question
A galvanized storage tank in a chemical process plant is used to contain de-aerated acid solution at 25C. Zinc reacts with acid according to the
A galvanized storage tank in a chemical process plant is used to contain de-aerated acid solution at 25C. Zinc reacts with acid according to the reaction:
Zn + 2H+ Zn2+ + H2
The rates of both oxidation and reduction half-reactions are controlled by activation polarization and the data are tabulated in
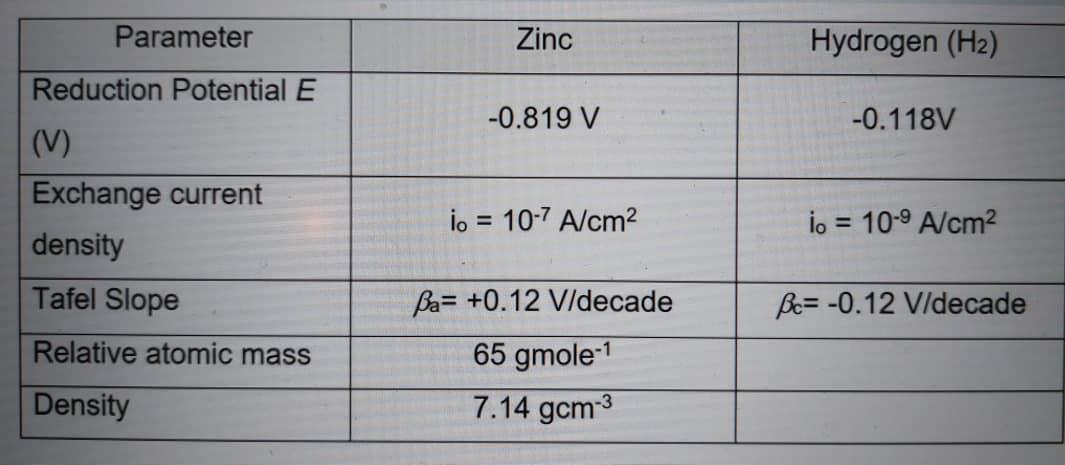
(a) Evaluate the concentration value of Zn2+. Given the standard reduction potential for zinc, E(Zn2+/Zn) = -0.76V.
(b) Determine the corrosion potential and corrosion current density of the system.
(c) Assess whether the zinc coating of 2.0mm thickness can last for 20 years of service before it corrodes completely.
(d) Qualitatively evaluate the effect of on cathodic reaction if this reaction is controlled by activation polarization at lower potential and becomes dominantly controlled by concentration polarization at high over potential. Justify your answer with schematic E-log i kinetics diagram.
\begin{tabular}{|l|c|c|} \hline \multicolumn{1}{|c|}{ Parameter } & Zinc & Hydrogen (H2) \\ \hline Reduction Potential & 0.819V & 0.118V \\ (V) & & \\ \hline Exchangecurrentdensity & i0=107A/cm2 & i0=109A/cm2 \\ \hline Tafel Slope & a=+0.12V/decade & c=0.12V/decade \\ \hline Relative atomic mass & 65gmole1 & \\ \hline Density & 7.14gcm3 & \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


