Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas enters a steady-flow adiabatic turbine at upstream conditions of 8 bar and 260 degc. The gas leaves the turbine at 1.5 bar and
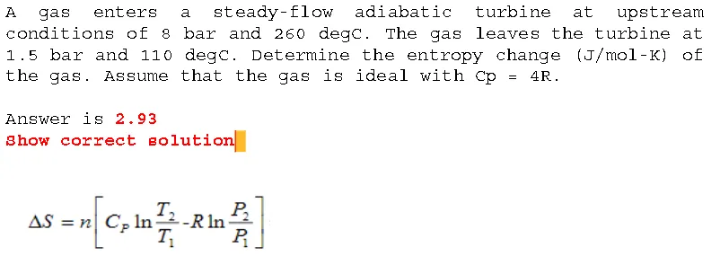 A gas enters a steady-flow adiabatic turbine at upstream conditions of 8 bar and 260 degc. The gas leaves the turbine at 1.5 bar and 110 dege. Determine the entropy change (J/mol-K) of the gas. Assume that the gas is ideal with Cp=4R. Answer is 2.93 show correct solution S=n[CPlnT1T2RlnP1P2]
A gas enters a steady-flow adiabatic turbine at upstream conditions of 8 bar and 260 degc. The gas leaves the turbine at 1.5 bar and 110 dege. Determine the entropy change (J/mol-K) of the gas. Assume that the gas is ideal with Cp=4R. Answer is 2.93 show correct solution S=n[CPlnT1T2RlnP1P2] Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


