Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas phase reaction will be carried out in a batch reactor 2A(g)--> 2B(g) + C(g) It was studied in a laboratory and the rate
A gas phase reaction will be carried out in a batch reactor 2A(g)--> 2B(g) + C(g)
It was studied in a laboratory and the rate of a chemical reaction was determined as a function of the conversion of reactant A. The laboratory measurements given show a chemical reaction rate as a function of conversion.
Pure A was fed to the reactor at 10 mol/L initial concentration.
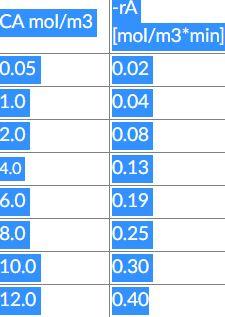
Initially determine the time to bring the reaction to a final concentration of A of 1.5 mol/m3.
-ra CA mol/m3 [mol/m3*min) 0.05 0.02 1.0 0.04 2.0 10.08 4.0 0.13 6.0 10.19 8.0 10.0 0.25 10.30 12.0 0.40Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


