Question
A gas stream contains 4mol% NH, and its ammonia content is to be reduced to 0.5mol% in a packed absorption tower at 293K and
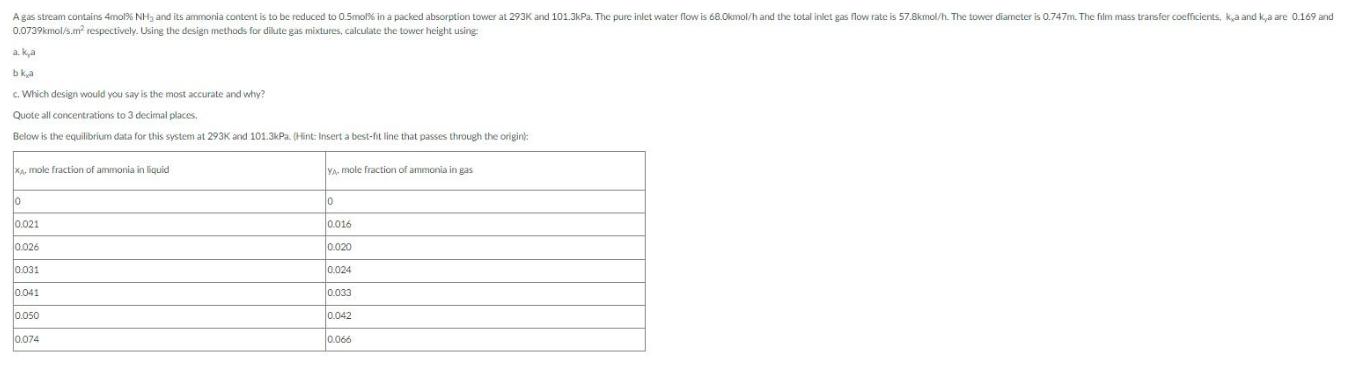
A gas stream contains 4mol% NH, and its ammonia content is to be reduced to 0.5mol% in a packed absorption tower at 293K and 101.3kPa. The pure inlet water flow is 68.0kmol/h and the total inlet gas flow rate is 57.8kmol/h. The tower diameter is 0.747m. The film mass transfer coefficients, ka and kya are 0.169 and 0.0739kmol/s.m respectively. Using the design methods for dilute gas mixtures, calculate the tower height using a.ka bka c. Which design would you say is the most accurate and why? Quote all concentrations to 3 decimal places. Below is the equilibrium data for this system at 293K and 101.3kPa. (Hint: Insert a best-fit line that passes through the origin): XA, mole fraction of ammonia in liquid YA, mole fraction of ammonia in gas 0 0 0.021 0.016 0.026 0.020 0.031 0.024 0.041 0.033 0.050 0.042 0.074 0.066
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Principles And Applications Of Mass Transfer
Authors: Jaime Benitez
4th Edition
1119785243, 978-1119785248
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



