Question
A mixture of 50 wt% ethanol and 50 wt % water which is saturated vapor is to be distilled at 101.3 kPa pressure to give
A mixture of 50 wt% ethanol and 50 wt % water which is saturated vapor is to be distilled at 101.3 kPa pressure to give a distillate containing 85 wt % ethanol and a bottom containing 3 wt % ethanol. The feed rate is 450 kg/h and a reflux ratio of 1.5 is to be used. Use equilibrium and enthalpy data from Appendix A.3 in Geankoplis. Note that data are given in wt fraction and kJ/kg. Use consistent units in plotting the enthalpy concentration and equilibrium data. a) Calculate the amount of distillate and bottoms. b) Calculate the number of theoretical trays needed. c) Calculate the condenser and reboiler loads 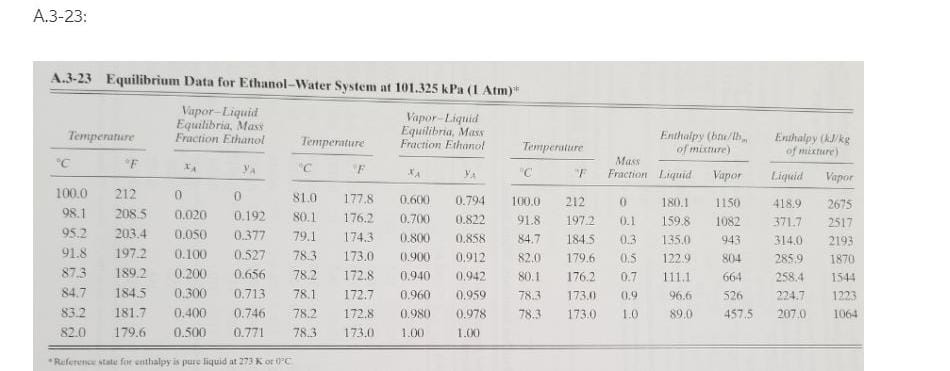
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


