Answered step by step
Verified Expert Solution
Question
1 Approved Answer
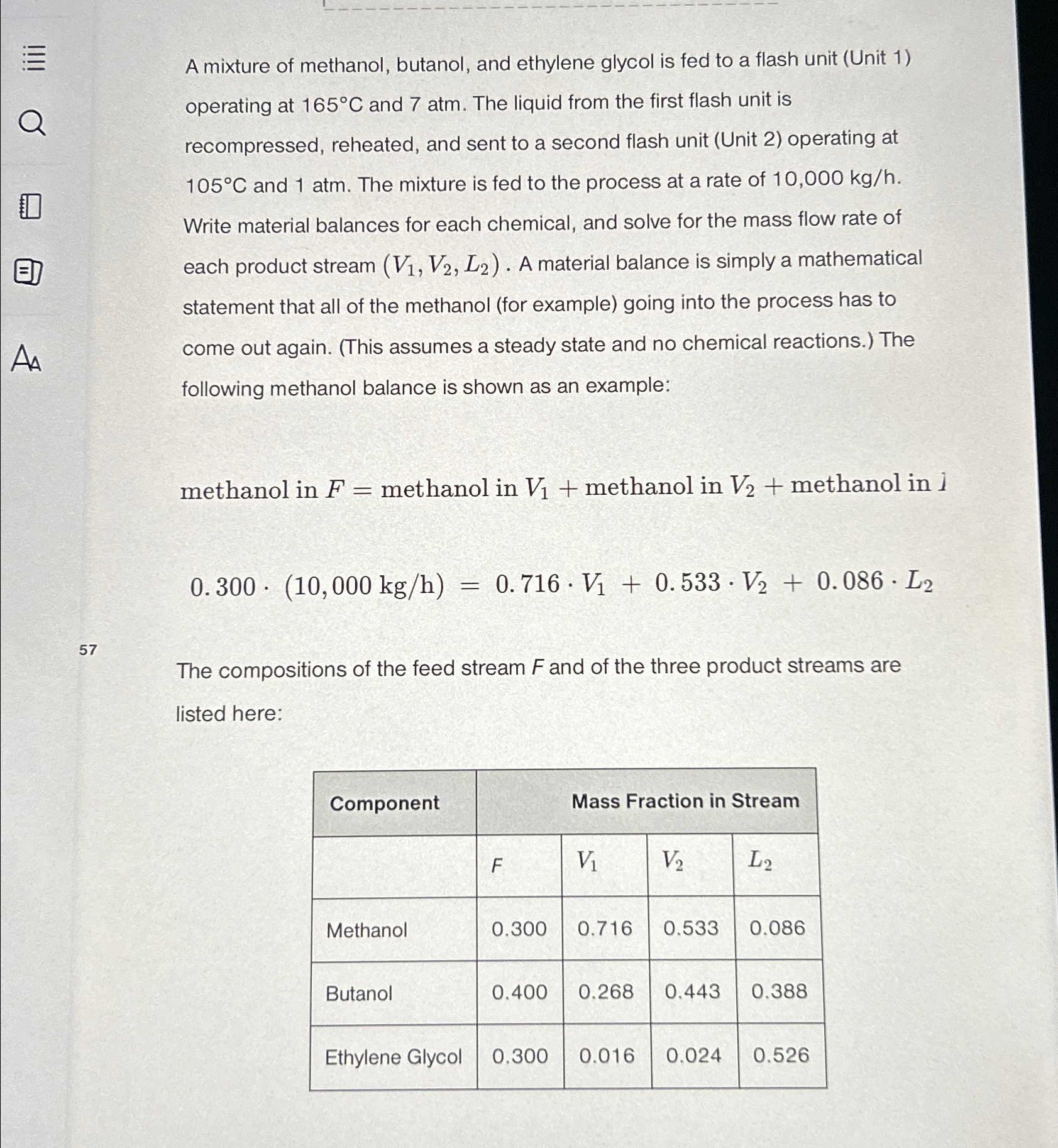
A mixture of methanol, butanol, and ethylene glycol is fed to a flash unit ( Unit 1 ) operating at 1 6 5 C and
A mixture of methanol, butanol, and ethylene glycol is fed to a flash unit Unit operating at and atm. The liquid from the first flash unit is recompressed, reheated, and sent to a second flash unit Unit operating at and atm. The mixture is fed to the process at a rate of Write material balances for each chemical, and solve for the mass flow rate of each product stream A material balance is simply a mathematical statement that all of the methanol for example going into the process has to come out again. This assumes a steady state and no chemical reactions. The following methanol balance is shown as an example:
methanol in methanol in methanol in methanol in i
The compositions of the feed stream and of the three product streams are listed here:
tableComponentMass Fraction in Stream
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


